Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. A gaseous chemical reaction below is taking place in a batch reactor, the volumetric flow rate at the end of the reaction was n-times
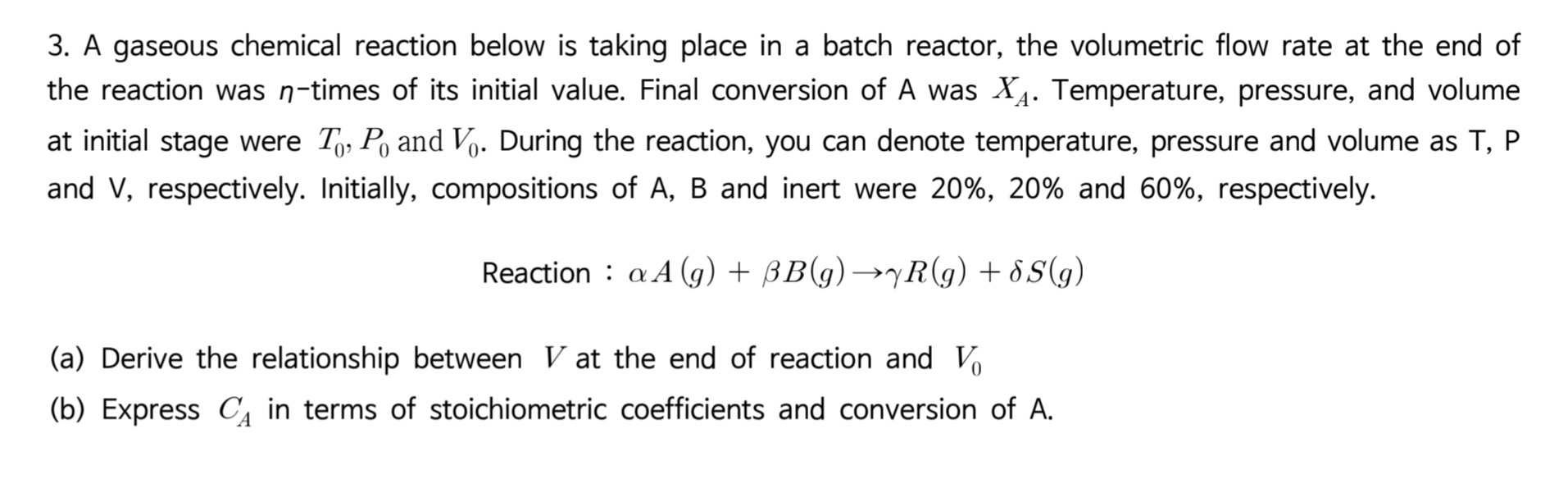 3. A gaseous chemical reaction below is taking place in a batch reactor, the volumetric flow rate at the end of the reaction was n-times of its initial value. Final conversion of A was XA. Temperature, pressure, and volume at initial stage were T0,P0 and V0. During the reaction, you can denote temperature, pressure and volume as T,P and V, respectively. Initially, compositions of A, B and inert were 20%,20% and 60%, respectively. Reaction : A(g)+B(g)R(g)+S(g) (a) Derive the relationship between V at the end of reaction and V0 (b) Express CA in terms of stoichiometric coefficients and conversion of A
3. A gaseous chemical reaction below is taking place in a batch reactor, the volumetric flow rate at the end of the reaction was n-times of its initial value. Final conversion of A was XA. Temperature, pressure, and volume at initial stage were T0,P0 and V0. During the reaction, you can denote temperature, pressure and volume as T,P and V, respectively. Initially, compositions of A, B and inert were 20%,20% and 60%, respectively. Reaction : A(g)+B(g)R(g)+S(g) (a) Derive the relationship between V at the end of reaction and V0 (b) Express CA in terms of stoichiometric coefficients and conversion of A Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


