Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. A liquid-process feed stream contains 99 mol-% of species 1 and 1 mol-% of impurity, species 2. The impurity level is to be
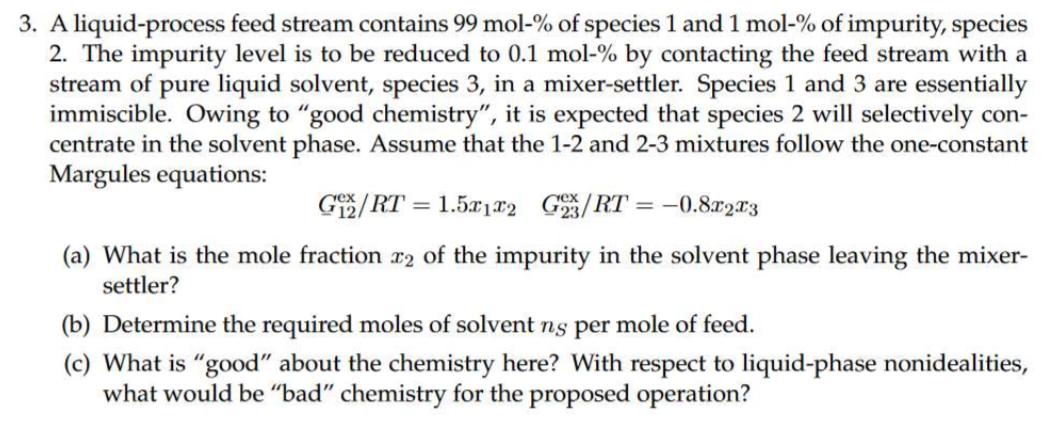
3. A liquid-process feed stream contains 99 mol-% of species 1 and 1 mol-% of impurity, species 2. The impurity level is to be reduced to 0.1 mol-% by contacting the feed stream with a stream of pure liquid solvent, species 3, in a mixer-settler. Species 1 and 3 are essentially immiscible. Owing to "good chemistry", it is expected that species 2 will selectively con- centrate in the solvent phase. Assume that the 1-2 and 2-3 mixtures follow the one-constant Margules equations: ex G/RT=1.5x1x2 G/RT=-0.8x2x3 (a) What is the mole fraction 2 of the impurity in the solvent phase leaving the mixer- settler? (b) Determine the required moles of solvent ns per mole of feed. (c) What is "good" about the chemistry here? With respect to liquid-phase nonidealities, what would be "bad" chemistry for the proposed operation?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The question presents a scenario involving a liquidliquid extraction process You have a feed stream species 1 with a small amount of impurity species 2 that is going to be contacted with a solvent spe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


