Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. As explained in Class, the transfer of heat from something hot to something cold is an irreversibility that results in an increase in
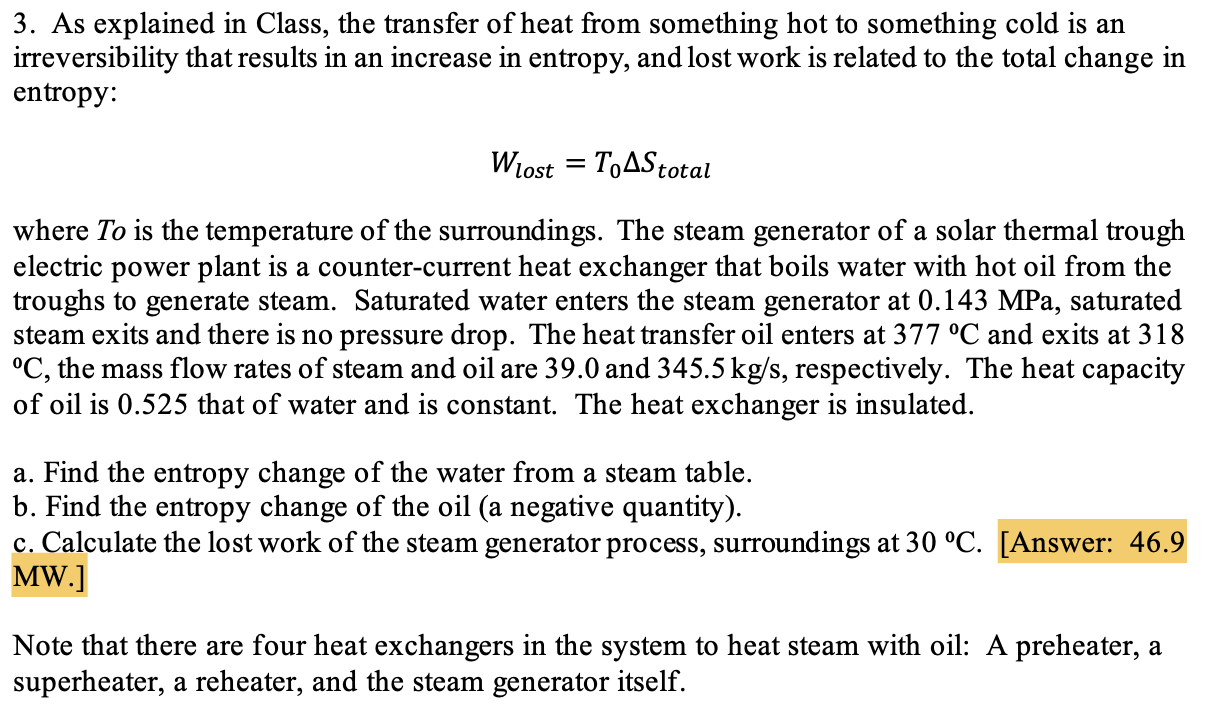
3. As explained in Class, the transfer of heat from something hot to something cold is an irreversibility that results in an increase in entropy, and lost work is related to the total change in entropy: = Wlost To Stotal where To is the temperature of the surroundings. The steam generator of a solar thermal trough electric power plant is a counter-current heat exchanger that boils water with hot oil from the troughs to generate steam. Saturated water enters the steam generator at 0.143 MPa, saturated steam exits and there is no pressure drop. The heat transfer oil enters at 377 C and exits at 318 C, the mass flow rates of steam and oil are 39.0 and 345.5 kg/s, respectively. The heat capacity of oil is 0.525 that of water and is constant. The heat exchanger is insulated. a. Find the entropy change of the water from a steam table. b. Find the entropy change of the oil (a negative quantity). c. Calculate the lost work of the steam generator process, surroundings at 30 C. [Answer: 46.9 MW.] Note that there are four heat exchangers in the system to heat steam with oil: A preheater, a superheater, a reheater, and the steam generator itself.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


