Question
3. Cobalt (Co) is the primary component of Co-based alloys used for biomedical implants. Cobalt has an HCP crystal structure, an atomic radius (R)
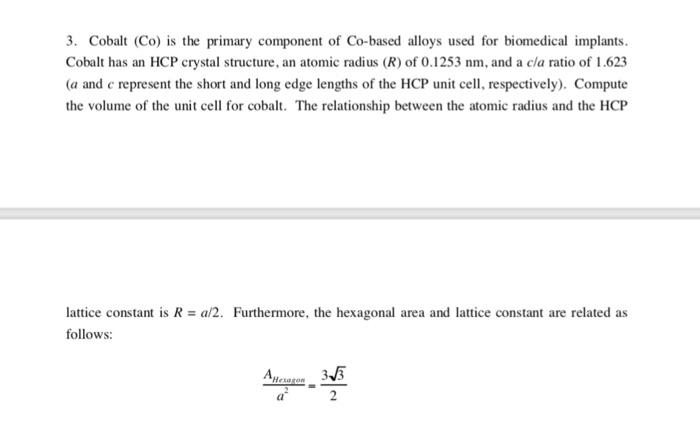
3. Cobalt (Co) is the primary component of Co-based alloys used for biomedical implants. Cobalt has an HCP crystal structure, an atomic radius (R) of 0.1253 nm, and a c/a ratio of 1.623 (a and c represent the short and long edge lengths of the HCP unit cell, respectively). Compute the volume of the unit cell for cobalt. The relationship between the atomic radius and the HCP lattice constant is R = a/2. Furthermore, the hexagonal area and lattice constant are related as follows: AHexagon 3-3
Step by Step Solution
3.27 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
The volume of an HCP lattice is calculated by the eq...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
9th edition
978-1118546895, 111854689X, 978-1118477700, 1118477707, 1118324579, 978-1118324578
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



