Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Consider the following data for two catalysts, A and B. The temperature is 25 C and the reaction occurs at standard conditions. a.
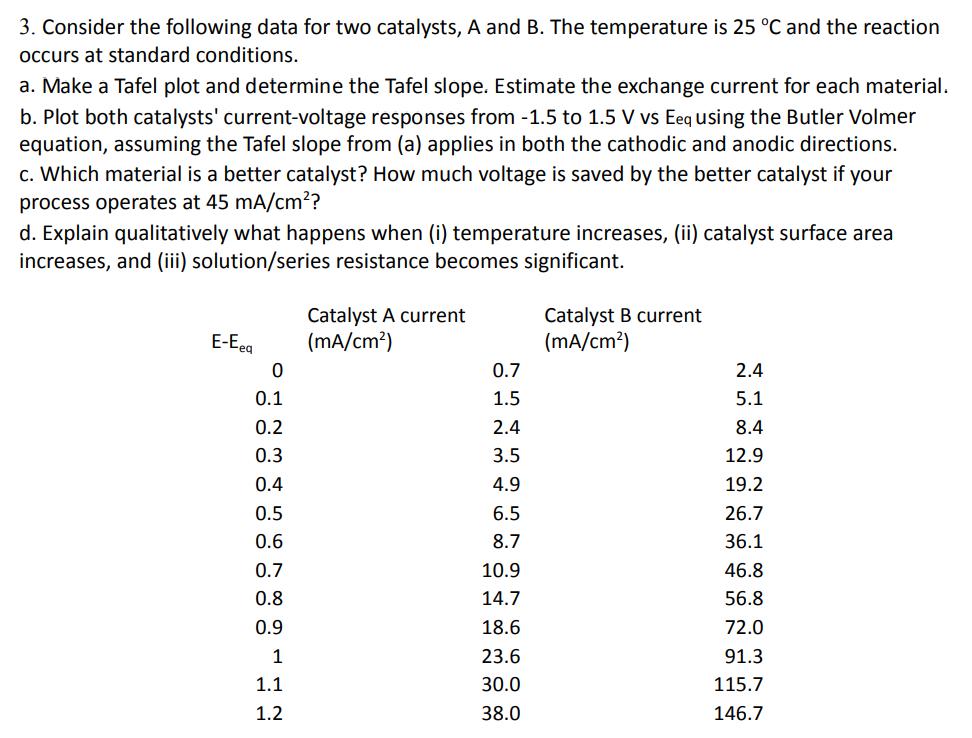
3. Consider the following data for two catalysts, A and B. The temperature is 25 C and the reaction occurs at standard conditions. a. Make a Tafel plot and determine the Tafel slope. Estimate the exchange current for each material. b. Plot both catalysts' current-voltage responses from -1.5 to 1.5 V vs Eeq using the Butler Volmer equation, assuming the Tafel slope from (a) applies in both the cathodic and anodic directions. c. Which material is a better catalyst? How much voltage is saved by the better catalyst if your process operates at 45 mA/cm? d. Explain qualitatively what happens when (i) temperature increases, (ii) catalyst surface area increases, and (iii) solution/series resistance becomes significant. E-Eeq Catalyst A current (mA/cm) Catalyst B current (mA/cm) 0 0.7 2.4 0.1 1.5 5.1 0.2 2.4 8.4 0.3 3.5 12.9 0.4 4.9 19.2 0.5 6.5 26.7 0.6 8.7 36.1 0.7 10.9 46.8 0.8 14.7 56.8 0.9 18.6 72.0 1 23.6 91.3 1.1 30.0 115.7 1.2 38.0 146.7
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Make a Tafel Plot and Determine the Tafel Slope Tafel Plot The Tafel plot is a graphical representation of the relationship between the overpotential E Eeq and the logarithm of the current density l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


