Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Cu-Ni Equilibrium Phase Diagram. 8 pts. Circle your answers. Composition (at% Ni) 80 100 20 60 40 o 1600 2800 1500 1455C Liquid 2600
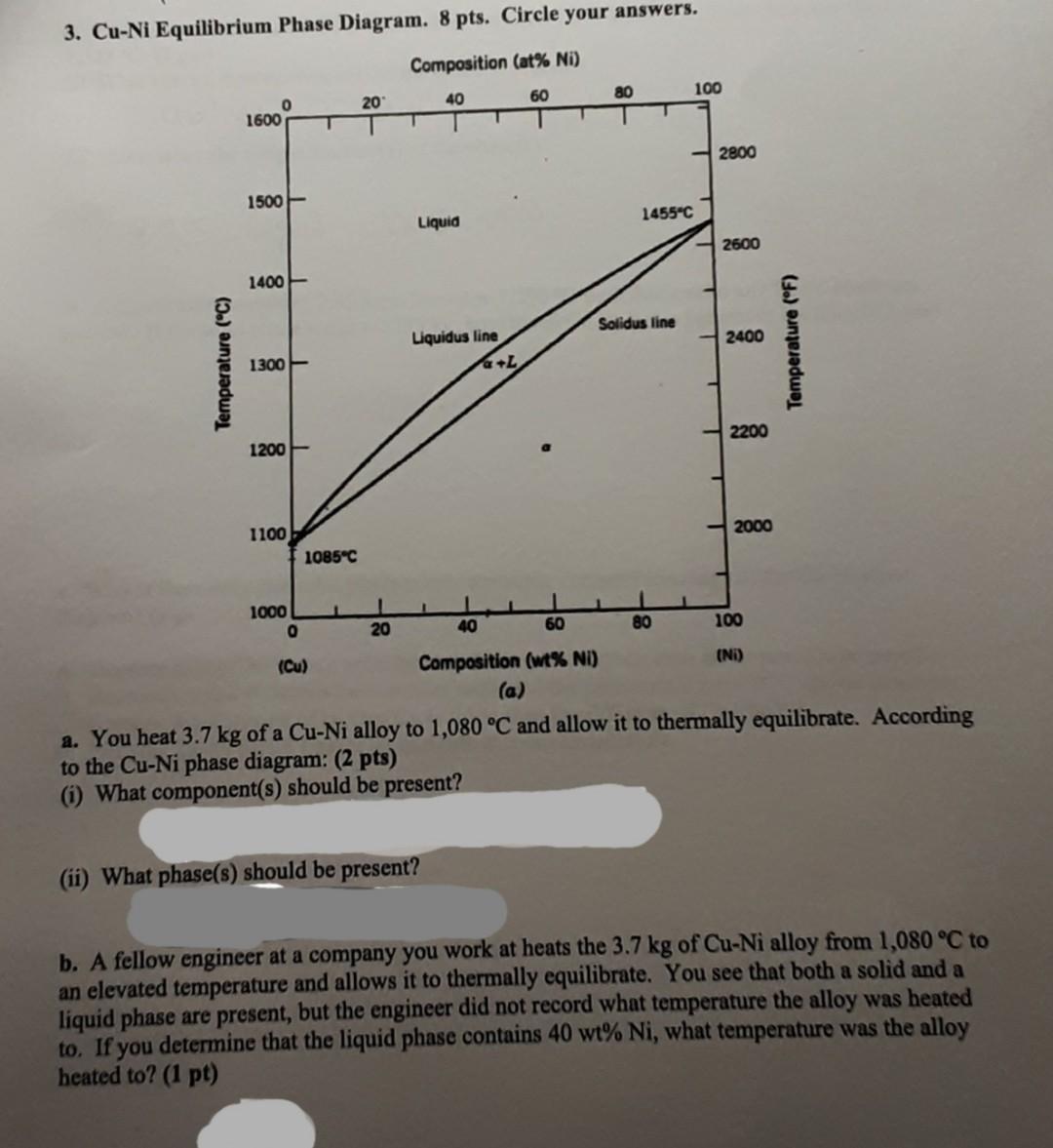

3. Cu-Ni Equilibrium Phase Diagram. 8 pts. Circle your answers. Composition (at% Ni) 80 100 20 60 40 o 1600 2800 1500 1455C Liquid 2600 1400 Solidus line 2400 Temperature (C) Liquidus line +L Temperature (F) 1300 2200 1200 2000 1100 1085C 1 1000 0 20 40 60 80 100 (Cu) Composition (wt% NI) (NI) (a) a. You heat 3.7 kg of a Cu-Ni alloy to 1,080 C and allow it to thermally equilibrate. According to the Cu-Ni phase diagram: (2 pts) (i) What component(s) should be present? (ii) What phase(s) should be present? b. A fellow engineer at a company you work at heats the 3.7 kg of Cu-Ni alloy from 1,080 C to an elevated temperature and allows it to thermally equilibrate. You see that both a solid and a liquid phase are present, but the engineer did not record what temperature the alloy was heated to. If you determine that the liquid phase contains 40 wt% Ni, what temperature was the alloy heated to? (1 pt) c. You determine that the 3.7-kg Cu-Ni alloy contains 1.665 kg of Cu. If you heat this alloy to 1,325 C: (2 pts) (i) What is/are the composition(s) of the phase(s) present? (ii) What is/are the weight fraction(s) of the phase(s)? a d. A Cu-Ni alloy weighing 2.45 kg is heated to 1,250 C such that a solid and liquid phase are present. If the solid phase weighs 0.735 kg, how much Cu (in kg) is present in the alloy? (2 pts) e. Why is there only one solid phase across the entire composition range of the Cu-Ni phase diagram? (1 pt) A. Because Cu and Ni form a solid that has fewer point defects than either pure Cu or pure Ni. B. Because Cu and Ni atoms have similar radii, and the pure metals form FCC crystal structures. Because the diffusion of Ni into Cu is faster than the diffusion of Cu into Ni. D. Because Cu-Ni alloys tend to be unstable at room temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


