Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Excited hydrogen. The energy levels of atomic hydrogen are given by the expres- sion E= -13.6/m electron volts, where n denotes the principal
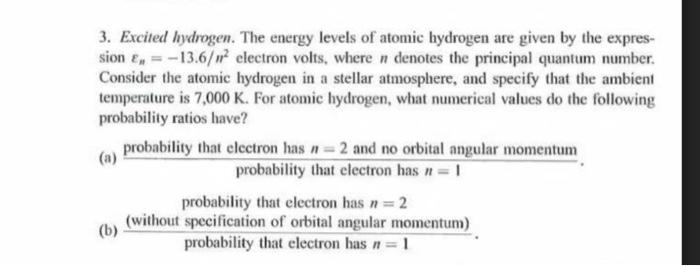
3. Excited hydrogen. The energy levels of atomic hydrogen are given by the expres- sion E= -13.6/m electron volts, where n denotes the principal quantum number. Consider the atomic hydrogen in a stellar atmosphere, and specify that the ambient temperature is 7,000 K. For atomic hydrogen, what numerical values do the following probability ratios have? (a) (b) probability that electron has n=2 and no orbital angular momentum probability that electron has n = 1 probability that electron has n = 2 (without specification of orbital angular momentum) probability that electron has n = 1
Step by Step Solution
★★★★★
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
a First lets calculate the energy levels of principal quantum numbers n1 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


