Answered step by step
Verified Expert Solution
Question
1 Approved Answer
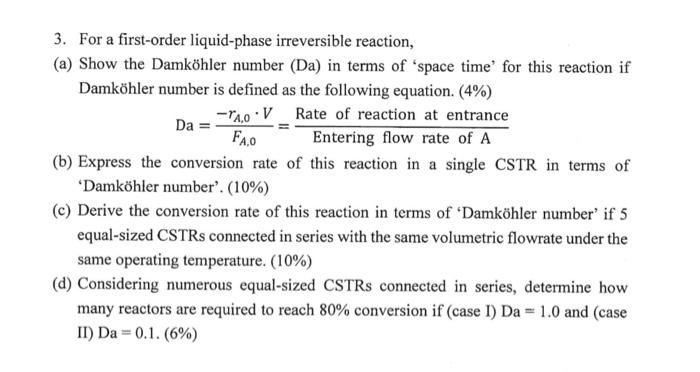
3. For a first-order liquid-phase irreversible reaction, (a) Show the Damkhler number (Da) in terms of 'space time for this reaction if Damkhler number is
3. For a first-order liquid-phase irreversible reaction,
(a) Show the Damkhler number (Da) in terms of 'space time for this reaction if Damkhler number is defined as the following equation. (4%)
- rA,0 V
Rate of reaction at entrance
Da =
FA.o
Entering flow rate of A
- Express the conversion rate of this reaction in a single CSTR in terms of 'Damkhler number'. (10%)
- Derive the conversion rate of this reaction in terms of "Damkhler number' if 5 equal-sized CSTRs connected in series with the same volumetric flowrate under the same operating temperature. (10%)
- Considering numerous equal-sized CSTRs connected in series, determine how many reactors are required to reach 80% conversion if (case I) Da = 1.0 and (case Il) Da = 0.1. (6%)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


