Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Gold forms complexes with both cyanide and thiosulphate. Write the balanced chemical reactions of the formation of the complexes using the two reagents. Compare






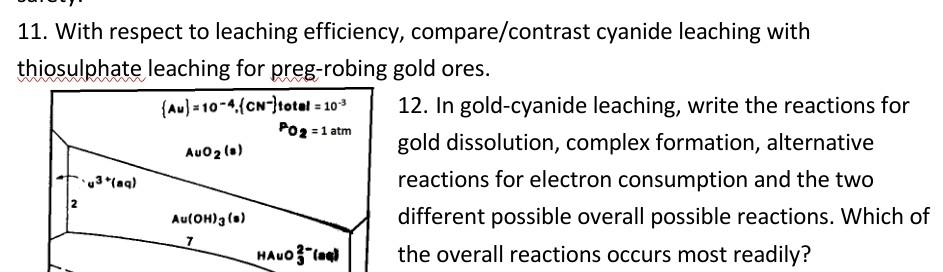
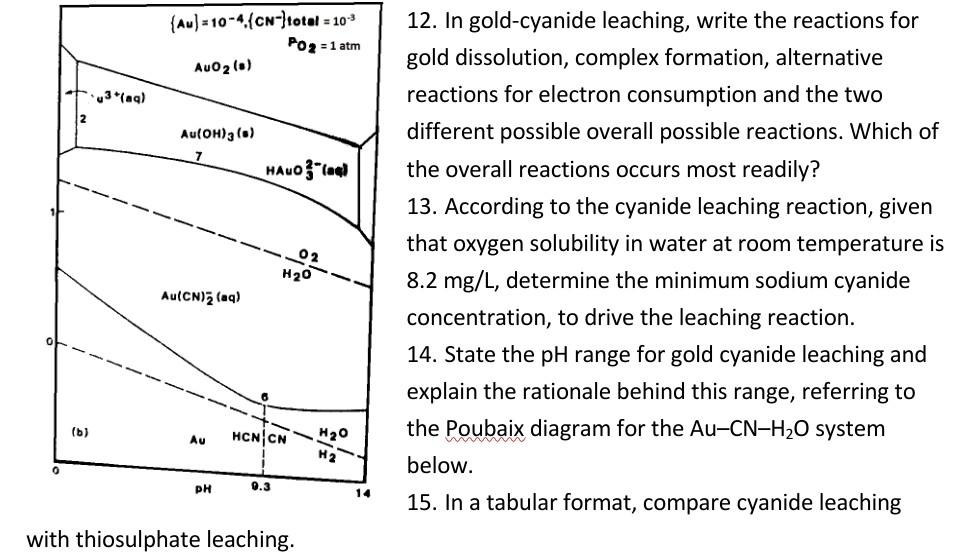

3. Gold forms complexes with both cyanide and thiosulphate. Write the balanced chemical reactions of the formation of the complexes using the two reagents. Compare the use of cyanide and thiosulphate as reagent for gold leaching cyanide and thiosulphate as reagent for gold leaching 4. Write balanced chemical reactions for (i) gold-cyanide leaching and (ii) gold-thiosulphate leaching 5. Based on the stoichiometry of the balanced equations, compare the dosages to leach equal amount of gold in cyanide leaching and in thiosulphate leaching. 6. A student attempts to carry out gold-cyanide leaching at pH 8. Comment on this experimental condition with respect to the process chemistry, leaching efficiency and safety. 7. In gold-cyanide leaching, write the redox reactions for gold dissolution, complex formation, and the two possible oxygen reduction reactions. 8. Complete and balance the sodium cyanide gold-leaching equation below - (i) with H2O2 in the product and (ii) without H2O2 in the product: Au + NaCN + O2 + 2H20 9. In the cyanide leaching test of an ore assaying 15 g/t Au, if the reaction proceeds by H2O2 production, what is the maximum quantity of the ore that can be leached in a 1 litre bottle filled with 50 mg/L sodium cyanide solution containing 0.00018 mol/L dissolved oxygen at room temperature? (6 marks) (Gram molar masses: Na = 23 g; C = 12 g; N = 14 g; O = 16 g; Au = 197 g). From your answer, comment on why oxygen from the air is not necessary in roll bottle leaching tests. 10. A student attempts to carry out gold-cyanide leaching at pH 8. Comment on this experimental condition with respect to the process chemistry, leaching efficiency and safety. 11. With respect to leaching efficiency, compare/contrast cyanide leaching with thiosulphate leaching for preg-robing gold ores. (Au) = 10-4 (CN-)total = 10-3 12. In gold-cyanide leaching, write the reactions for Po 2 = 1 atm AuOz (0) gold dissolution, complex formation, alternative 3(aq) reactions for electron consumption and the two Au(OH)3 (0) different possible overall possible reactions. Which of HALO- the overall reactions occurs most readily? 7 (Au) = 10-4 (CN-)total = 10-9 PO2 = 1 atm AuOz (5) 3(aq) 2 Au(OH)3 (6) HALO "la 02 12. In gold-cyanide leaching, write the reactions for gold dissolution, complex formation, alternative reactions for electron consumption and the two different possible overall possible reactions. Which of the overall reactions occurs most readily? 13. According to the cyanide leaching reaction, given that oxygen solubility in water at room temperature is 8.2 mg/L, determine the minimum sodium cyanide concentration, to drive the leaching reaction. 14. State the pH range for gold cyanide leaching and explain the rationale behind this range, referring to the Poubaix diagram for the Au-CN-H2O system below. 15. In a tabular format, compare cyanide leaching H20 Au(CN)2 (aq) 0 (b) Au HCNCN H2O H2 PH 14 with thiosulphate leaching. 16. It is not possible to dissolve gold in an acid no matter how concentrated. Explain. 17. Is it possible to dissolve gold in a very concentrated acid? Yes or no. Explain your choice. IL
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


