Question
3) How many moles and how many atoms of zinc are in a sample weighing 34.9 g? -25 A) 0.533 mol, 8.85 x107 B)
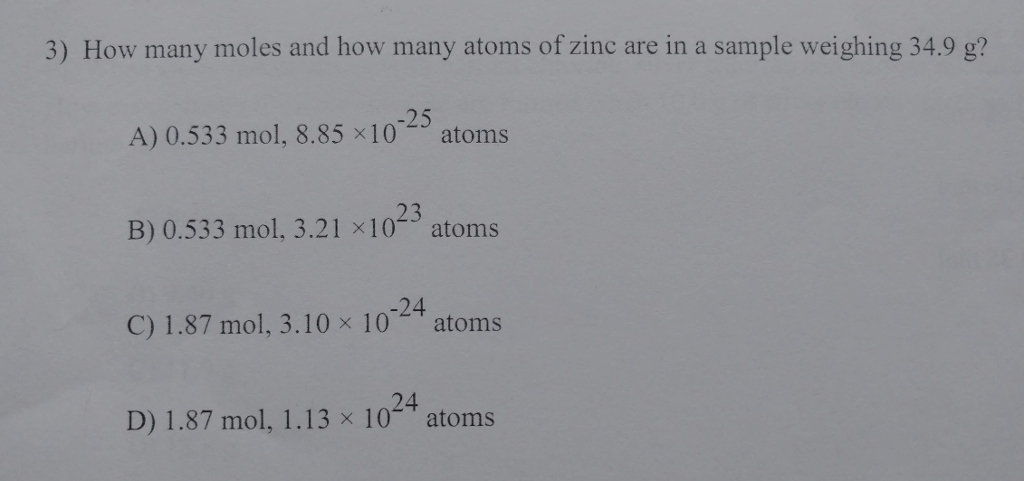
3) How many moles and how many atoms of zinc are in a sample weighing 34.9 g? -25 A) 0.533 mol, 8.85 x107 B) 0.533 mol, 3.21 1023 atoms atoms -24 C) 1.87 mol, 3.10 10 atoms 24 D) 1.87 mol, 1.13 x 10 atoms
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Molar mass of Zn 6538 gmol Given mass of Zn 349 gm m...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
A Survey of Mathematics with Applications
Authors: Allen R. Angel, Christine D. Abbott, Dennis Runde
10th edition
134112105, 134112342, 9780134112343, 9780134112268, 134112261, 978-0134112107
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



