Answered step by step
Verified Expert Solution
Question
1 Approved Answer
~ 3. Inspired by experiments of Guironnet et al. and Hartwig et al., Ziqiu Chen modeled the kinetics of a polymer upcycling strategy called
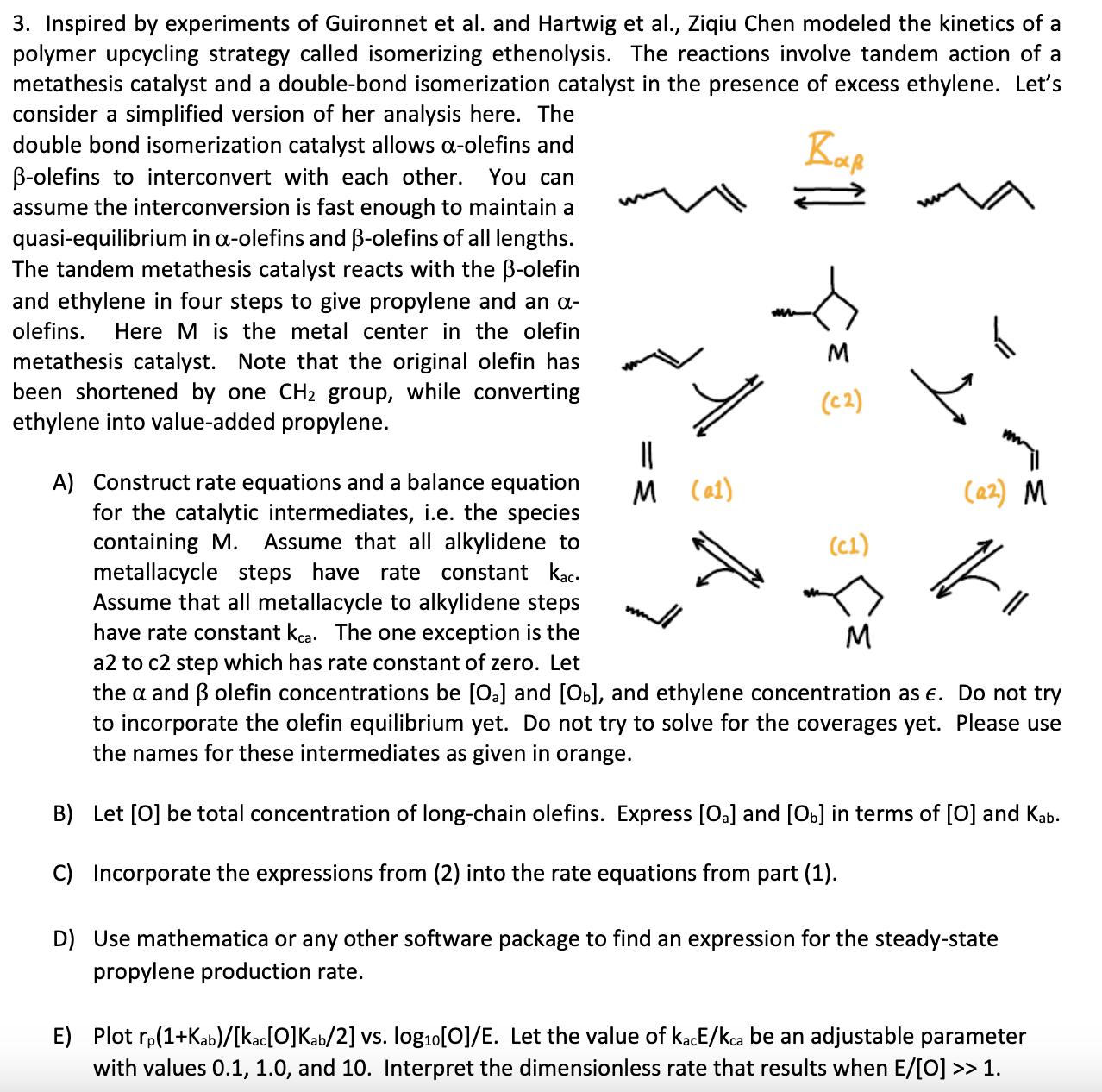
~ 3. Inspired by experiments of Guironnet et al. and Hartwig et al., Ziqiu Chen modeled the kinetics of a polymer upcycling strategy called isomerizing ethenolysis. The reactions involve tandem action of a metathesis catalyst and a double-bond isomerization catalyst in the presence of excess ethylene. Let's consider a simplified version of her analysis here. The double bond isomerization catalyst allows a-olefins and -olefins to interconvert with each other. You can assume the interconversion is fast enough to maintain a quasi-equilibrium in a-olefins and -olefins of all lengths. The tandem metathesis catalyst reacts with the -olefin and ethylene in four steps to give propylene and an - olefins. Here M is the metal center in the olefin metathesis catalyst. Note that the original olefin has been shortened by one CH2 group, while converting ethylene into value-added propylene. Y M (C2) M (a1) (a2) M (C1) >= A) Construct rate equations and a balance equation for the catalytic intermediates, i.e. the species containing M. Assume that all alkylidene to metallacycle steps have rate constant kac. Assume that all metallacycle to alkylidene steps have rate constant kca. The one exception is the a2 to c2 step which has rate constant of zero. Let the a and olefin concentrations be [Oa] and [Ob), and ethylene concentration as . Do not try to incorporate the olefin equilibrium yet. Do not try to solve for the coverages yet. Please use the names for these intermediates as given in orange. M B) Let [O] be total concentration of long-chain olefins. Express [Oa] and [b] in terms of [O] and Kab. C) Incorporate the expressions from (2) into the rate equations from part (1). D) Use mathematica or any other software package to find an expression for the steady-state propylene production rate. E) Plot rp(1+Kab)/[kac[0]Kab/2] vs. log10 [O]/E. Let the value of KacE/kca be an adjustable parameter with values 0.1, 1.0, and 10. Interpret the dimensionless rate that results when E/[O] >> 1.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


