Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. It took 116 hours to produce 603 g of metal X by performing electrolysis on molten XCl3 with a current of 2.00 A. Calculate
3. It took 116 hours to produce 603 g of metal X by performing electrolysis on molten XCl3 with a current of 2.00 A. Calculate the molar mass of X.
I need this done in a linear method.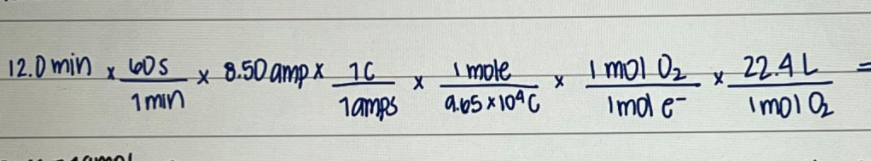
just like this picture i need it done. that is a example ^^^
12.0 min X 605 x8.50 amp x 10 1min 1amps TOWIDT X Imole 9.65104C X 1 mol 0 x 22.4L Imol e Imo10 XStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


