Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Most proteins are covalently modified in the ER. [Pages 507-509] a) Figure 15-23 illustrates the glycosylation of a protein specific asparagine(ASN) as the
![3. Most proteins are covalently modified in the ER. [Pages 507-509]a) Figure 15-23 illustrates the glycosylation of a protei](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/05/62947574bc781_1653896562952.jpg)
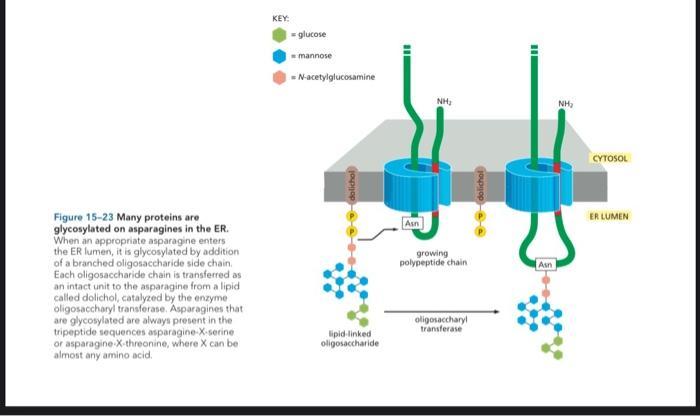
3. Most proteins are covalently modified in the ER. [Pages 507-509] a) Figure 15-23 illustrates the glycosylation of a protein specific asparagine(ASN) as the growing peptide chain enters the ER lumen. How might this co-translational process assist in recognizing which ASN to glycosylate? b) Glycosylation involves the attachment of a pre-made oligonucleotides to a protein making it into a glycoprotein. From an energy efficiency and quality control perspective, what is the advantage of attaching a preassembled oligonucleotide instead of building it on the protein block by block (by the sequential addition of monosaccharides)? Figure 15-23 Many proteins are glycosylated on asparagines in the ER. When an appropriate asparagine enters the ER lumen, it is glycosylated by addition of a branched oligosaccharide side chain Each oligosaccharide chain is transferred as an intact unit to the asparagine from a lipid called dolichol, catalyzed by the enzyme oligosaccharyl transferase. Asparagines that are glycosylated are always present in the tripeptide sequences asparagine-X-serine or asparagine-X-threonine, where X can be almost any amino acid. KEY: -glucose mannose N-acetylglucosamine dalichol lipid-linked oligosaccharide Asn NH growing polypeptide chain oligosaccharyl transferase dolichol 11 Asn 0 NH CYTOSOL ER LUMEN
Step by Step Solution
★★★★★
3.37 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
a Glycosylation of proteins in ER is Nlinked glycosylation not Olin...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


