Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Nucleation Following is an expression for the Gibbs free energy required to nucleate a spherical solid of radius r in a liquid at
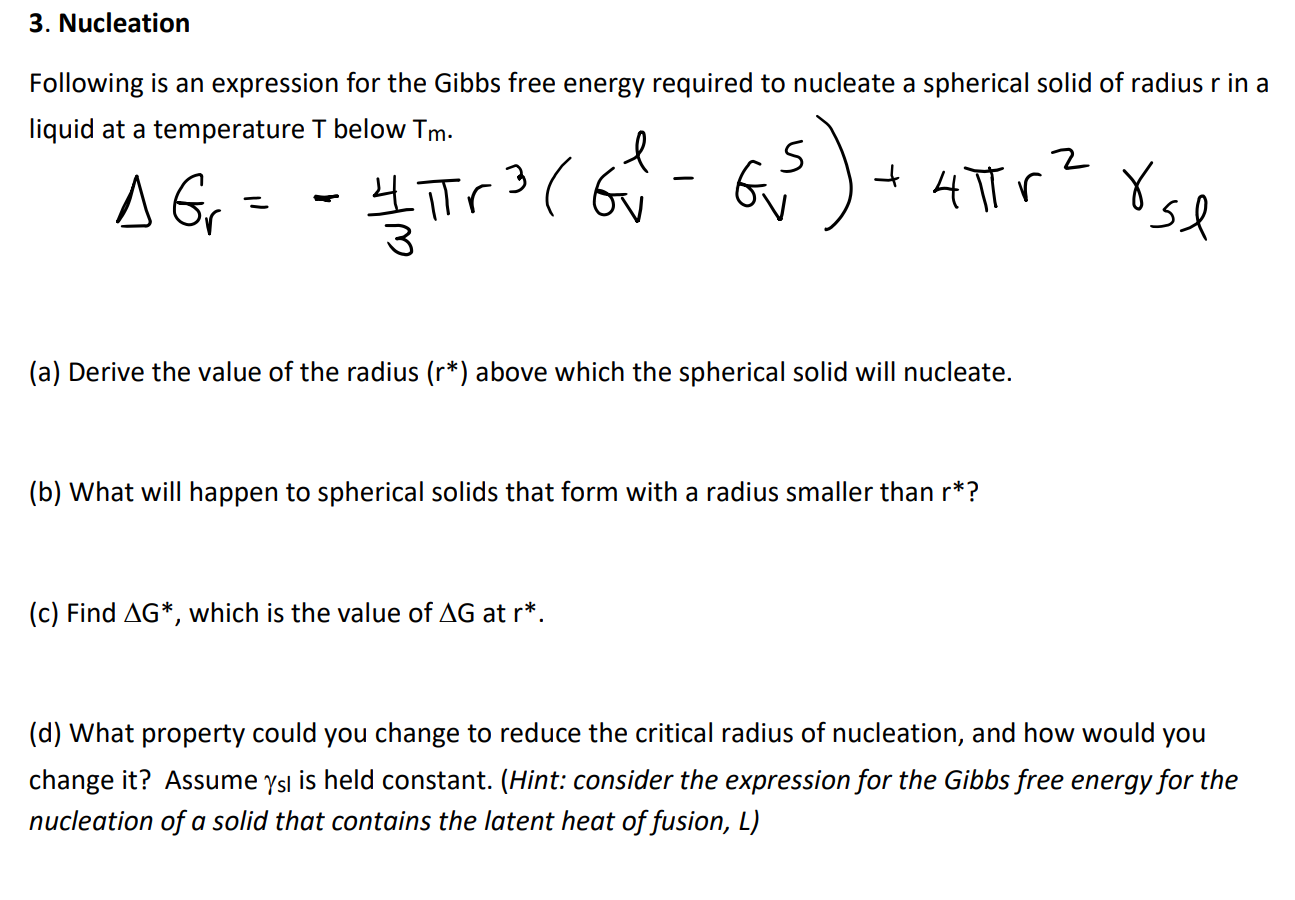
3. Nucleation Following is an expression for the Gibbs free energy required to nucleate a spherical solid of radius r in a liquid at a temperature T below Tm. A G = - 3 S 141 |r (61 - 6) + 4r 8se G (a) Derive the value of the radius (r*) above which the spherical solid will nucleate. (b) What will happen to spherical solids that form with a radius smaller than r*? (c) Find AG*, which is the value of AG at r*. (d) What property could you change to reduce the critical radius of nucleation, and how would you change it? Assume ysl is held constant. (Hint: consider the expression for the Gibbs free energy for the nucleation of a solid that contains the latent heat of fusion, L)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


