Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A hypothetical system contains three different molecules, each of which is capable of having the energies: = 0, 1, 2, 3, 4, ( 2.410-4
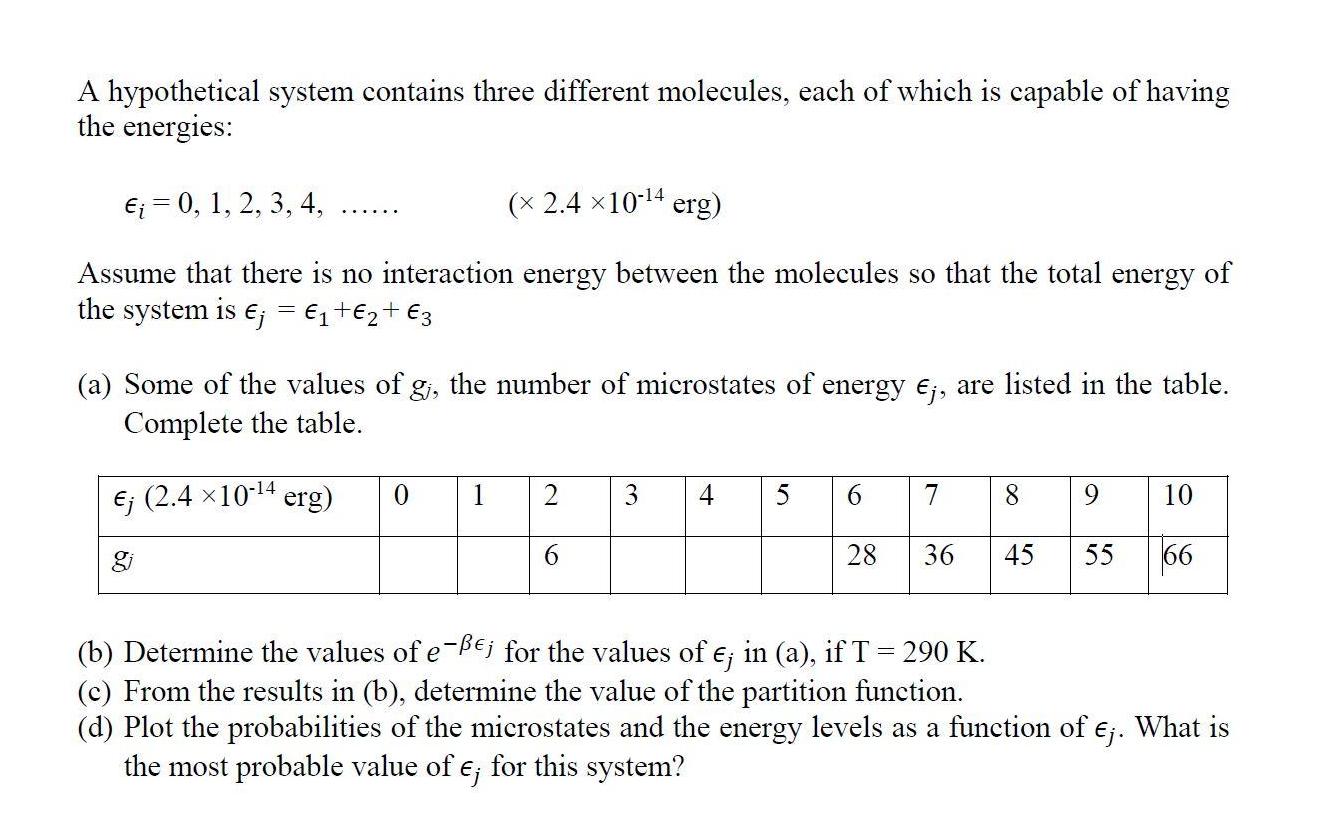
A hypothetical system contains three different molecules, each of which is capable of having the energies: = 0, 1, 2, 3, 4, ( 2.410-4 erg) Assume that there is no interaction energy between the molecules so that the total energy of the system is ; = + + 3 (a) Some of the values of g, the number of microstates of energy Ej, are listed in the table. Complete the table. (2.4 10-4 erg) 0 1 2 6 3 4 5 6 7 8 9 10 28 36 45 55 66 (b) Determine the values of e-Bej for the values of e; in (a), if T = 290 K. (c) From the results in (b), determine the value of the partition function. (d) Plot the probabilities of the microstates and the energy levels as a function of . What is the most probable value of e; for this system? A hypothetical system contains three different molecules, each of which is capable of having the energies: = 0, 1, 2, 3, 4, ( 2.410-4 erg) Assume that there is no interaction energy between the molecules so that the total energy of the system is ; = + + 3 (a) Some of the values of g, the number of microstates of energy Ej, are listed in the table. Complete the table. (2.4 10-4 erg) 0 1 2 6 3 4 5 6 7 8 9 10 28 36 45 55 66 (b) Determine the values of e-Bej for the values of e; in (a), if T = 290 K. (c) From the results in (b), determine the value of the partition function. (d) Plot the probabilities of the microstates and the energy levels as a function of . What is the most probable value of e; for this system?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


