Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Construct the phase diagram for the system, and draw in the tie-lines. 4. An aqueous solution contains 46% by weight of ethyl alcohol. Using the
Construct the phase diagram for the system, and draw in the tie-lines.
4. An aqueous solution contains 46% by weight of ethyl alcohol. Using the diagram of the preceding problem, find how much alcohol would be extracted from 25 g of this solution by 100 g of C6H6.
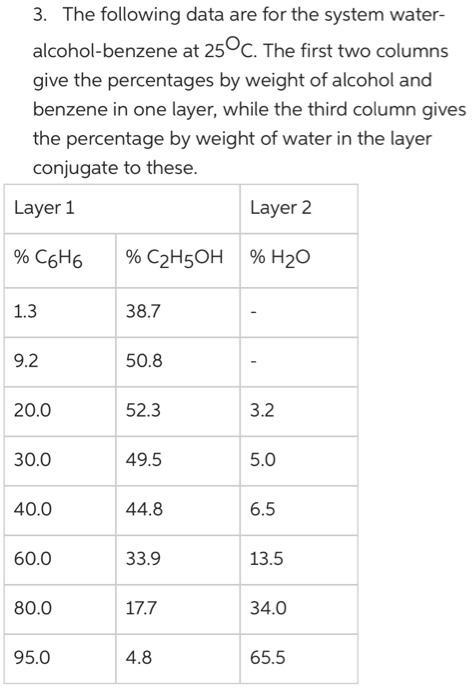
3. The following data are for the system water- alcohol-benzene at 25C. The first two columns give the percentages by weight of alcohol and benzene in one layer, while the third column gives the percentage by weight of water in the layer conjugate to these. Layer 1 Layer 2 % C6H6 % C2H5OH % H20 1.3 38.7 9.2 50.8 20.0 52.3 3.2 30.0 49.5 5.0 40.0 44.8 6.5 60.0 33.9 13.5 80.0 17.7 34.0 95.0 4.8 65.5
Step by Step Solution
★★★★★
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
using the mass and component balance of ethyl alcohol over the system ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


