Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. The rate law of the dissolution of MnO2 in HBr is assumed of the form Thor = kCABO An experimental data was listed in
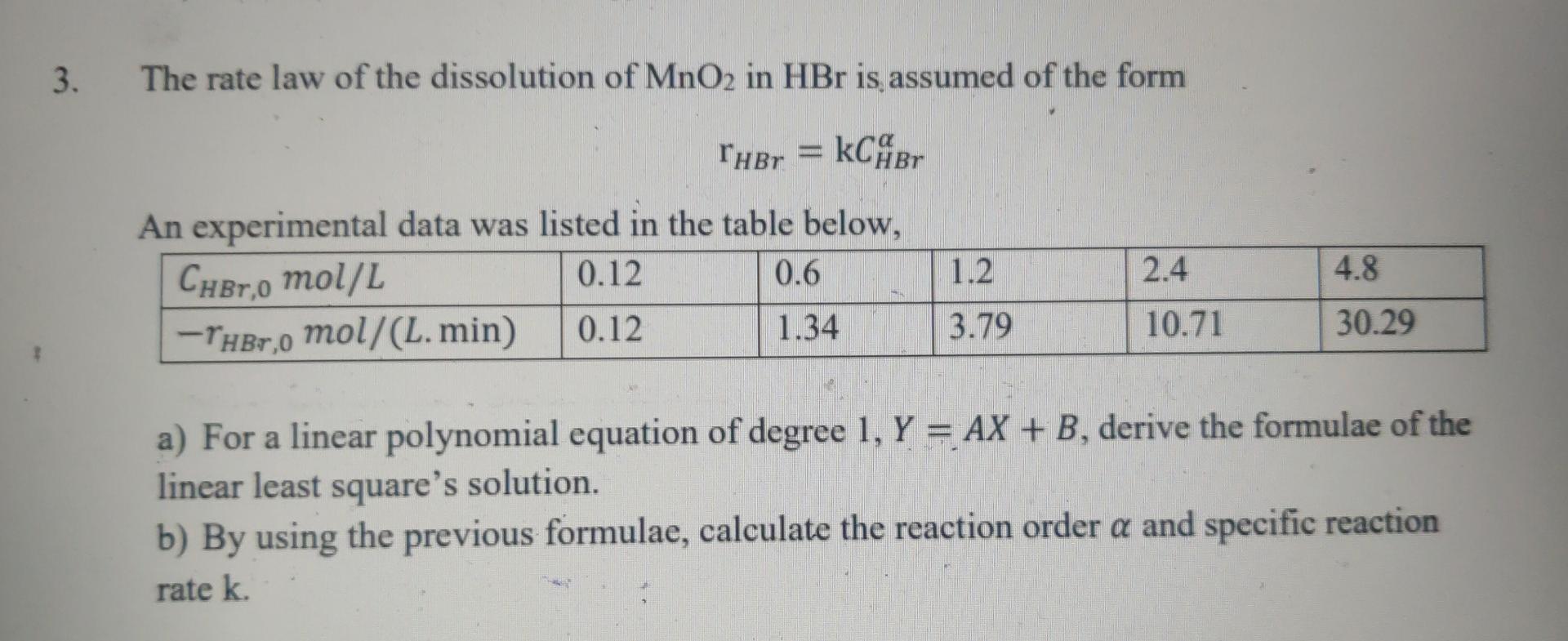
3. The rate law of the dissolution of MnO2 in HBr is assumed of the form Thor = kCABO An experimental data was listed in the table below, Cher,0 mol/L 0.12 0.6 -Ther,0 mol/(L. min) 0.12 1.34 1.2 2.4 4.8 3.79 10.71 30.29 a) For a linear polynomial equation of degree 1, Y = AX + B, derive the formulae of the linear least square's solution. b) By using the previous formulae, calculate the reaction order a and specific reaction rate k
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


