Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Toluene hydrogenation reaction was carried out over Ni/Al2O3 catalyst which has the particle and solid density of 1.25 g/cm and 3.25 g/cm, respectively.
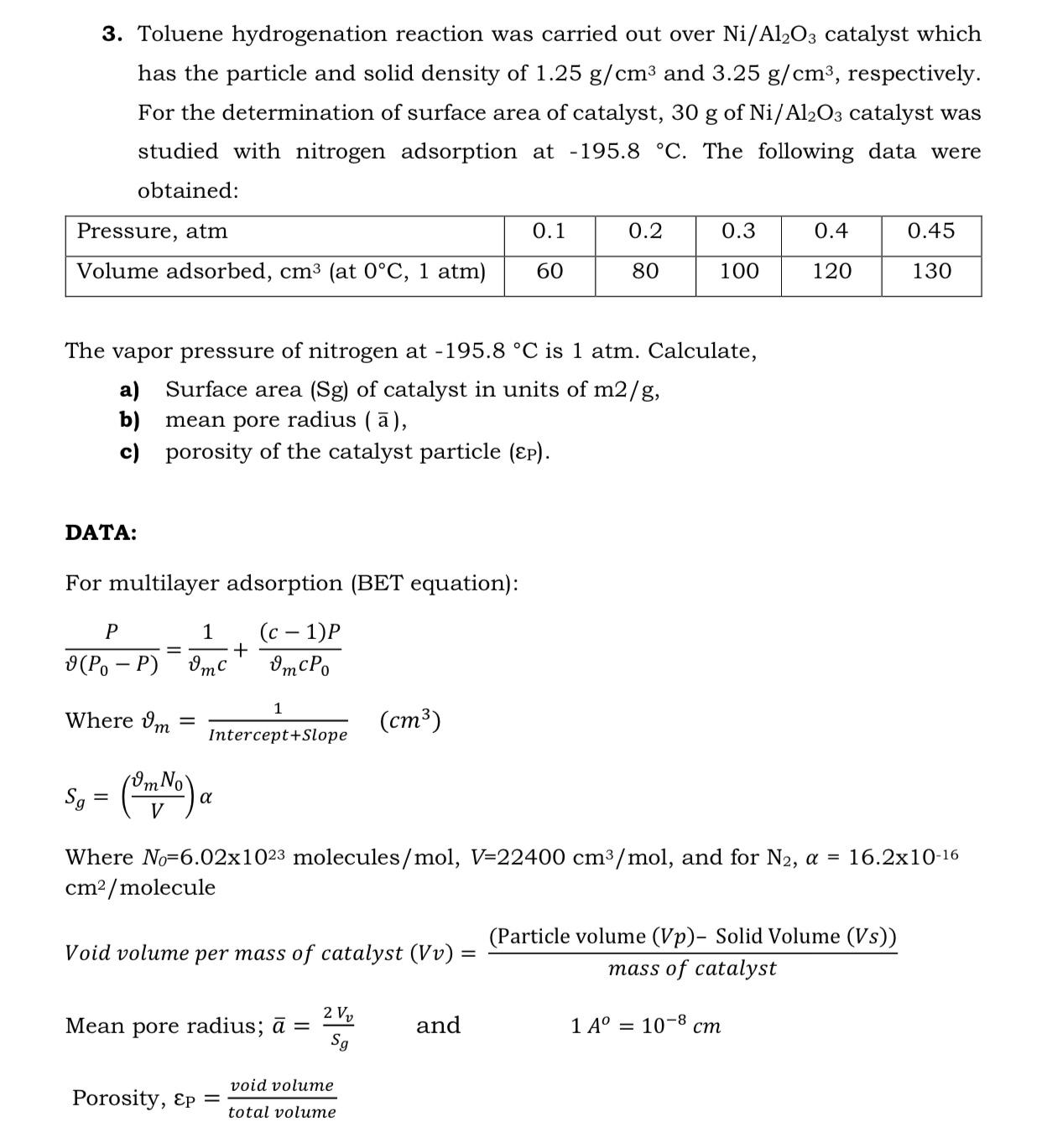
3. Toluene hydrogenation reaction was carried out over Ni/Al2O3 catalyst which has the particle and solid density of 1.25 g/cm and 3.25 g/cm, respectively. For the determination of surface area of catalyst, 30 g of Ni/Al2O3 catalyst was studied with nitrogen adsorption at -195.8 C. The following data were obtained: Pressure, atm Volume adsorbed, cm (at 0C, 1 atm) DATA: For multilayer adsorption (BET equation): (c 1)P mcPo The vapor pressure of nitrogen at -195.8 C is 1 atm. Calculate, a) Surface area (Sg) of catalyst in units of m2/g, b) mean pore radius (), c) porosity of the catalyst particle (EP). P 9(Po-P) = 1 vmc Where m = + 1 Intercept+Slope Mean pore radius; = Porosity, Ep = (cm) void volume total volume 0.1 60 0.2 80 and 0.3 100 Sg = (Om No) a Where No-6.02x1023 molecules/mol, V=22400 cm/mol, and for N2, a = 16.2x10-16 cm/molecule Void volume per mass of catalyst (Vv) = 2 V Sg 0.4 120 (Particle volume (Vp)- Solid Volume (Vs)) mass of catalyst 1 A= 10-8 cm 0.45 130
Step by Step Solution
★★★★★
3.46 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


