Question
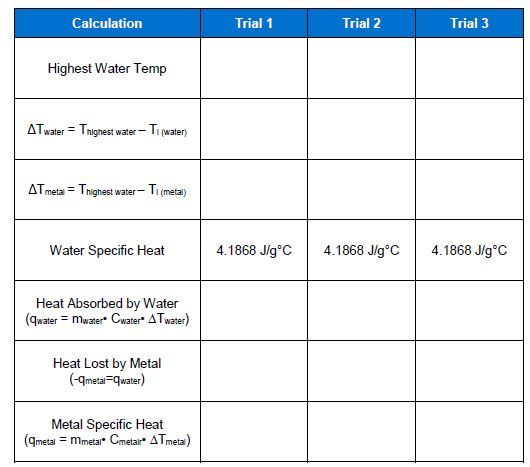
3. What is the average specific heat capacity of the unknownmetal for the three trials? 4. What is the unknown metal in Experiment 1? Use
3. What is the average specific heat capacity of the unknownmetal for the three trials?
4. What is the unknown metal in Experiment 1? Use Table 1 (inthe Introduction) for reference.
5. A metal sample weighing 43.5 g at a temperature of 100.0 ?Cwas placed in 39.9 g ofwater in a calorimeter at 25.1?C. Atequilibrium, the temperature of the water and metalwas33.5?C.a.What was ?T for the water? (?T = Tfinal - Tinitial)b.What was ?T for the metal?
6. Using the specific heat of water (4.184 J/g?C), calculate howmuch heat flowedinto the water. (Show all your work for thiscalculation)
7. Calculate the specific heat of the metal. (Show all your workfor this calculation
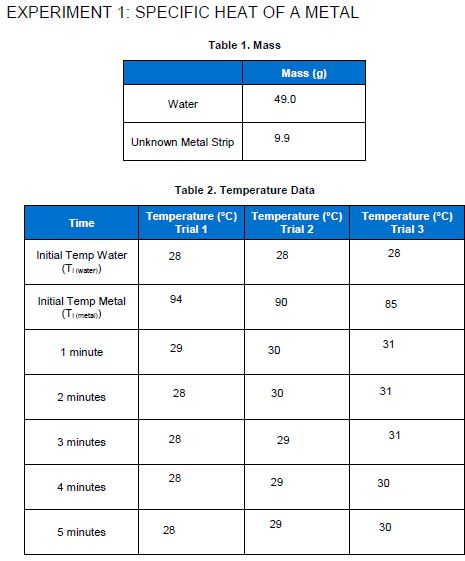
EXPERIMENT 1: SPECIFIC HEAT OF A METAL Time Initial Temp Water (Ti(water)) Initial Temp Metal (Ti(metal)) 1 minute 2 minutes 3 minutes 4 minutes 5 minutes. Water Unknown Metal Strip 28 94 29 Table 2. Temperature Data Temperature (C) Temperature (C) Temperature (C) Trial 1 Trial 2 Trial 3 28 28 28 Table 1. Mass 28 28 Mass (g) 49.0 9.9 28 90 30 30 29 29 29 85 31 31 31 30 30
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Trial 1 Trial Heat lost by melal Heat mm pm 4Tm mw pw 4 Tw Cp...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


