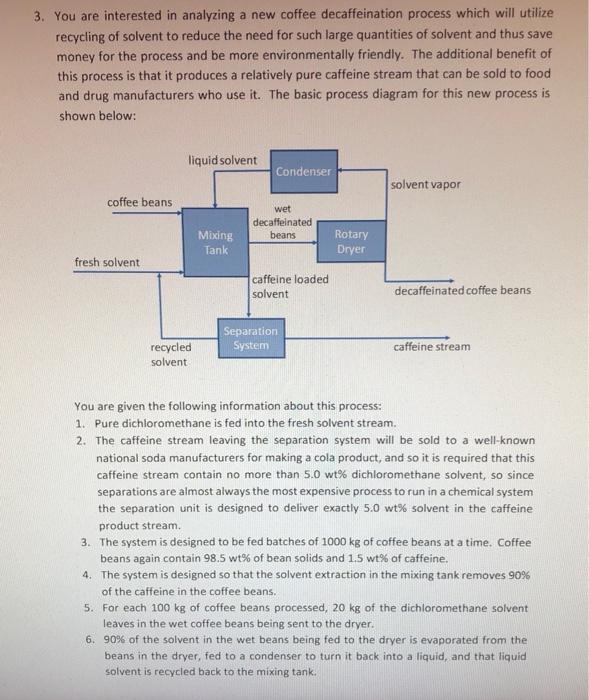
3. You are interested in analyzing a new coffee decaffeination process which will utilize recycling of solvent to reduce the need for such large quantities of solvent and thus save money for the process and be more environmentally friendly. The additional benefit of this process is that it produces a relatively pure caffeine stream that can be sold to food and drug manufacturers who use it. The basic process diagram for this new process is shown below: You are given the following information about this process: 1. Pure dichloromethane is fed into the fresh solvent stream. 2. The caffeine stream leaving the separation system will be sold to a well-known national soda manufacturers for making a cola product, and so it is required that this caffeine stream contain no more than 5.0wt% dichloromethane solvent, so since separations are almost always the most expensive process to run in a chemical system the separation unit is designed to deliver exactly 5.0wt solvent in the caffeine product stream. 3. The system is designed to be fed batches of 1000kg of coffee beans at a time. Coffee beans again contain 98.5wt% of bean solids and 1.5 wt % of caffeine. 4. The system is designed so that the solvent extraction in the mixing tank removes 90% of the caffeine in the coffee beans. 5. For each 100kg of coffee beans processed, 20kg of the dichloromethane solvent leaves in the wet coffee beans being sent to the dryer. 6. 90% of the solvent in the wet beans being fed to the dryer is evaporated from the beans in the dryer, fed to a condenser to turn it back into a liquid, and that liquid solvent is recycled back to the mixing tank. 7. The primary solvent stream being fed to mixing tank, composed of the fresh solvent and the recycled solvent from the separation unit, is tested and found to be 95wt% dichloromethane solvent. 8. The solvent leaving the mixing tank that contains the extracted caffeine is tested and found to be 88 wt\% dichloromethane solvent. Your assignment is to answer the following two questions: 1. How much fresh solvent must be purchased and fed to the process for each 1000kg batch of coffee beans processed? 2. In order to constantly monitor if the separation unit is operating properly, you are going to put a composition analyzer on the recycled solvent stream leaving it. What should the composition of the recycled solvent stream be for this process if it is running as designed? Include your labeled diagram. Indicate system boundaries and show degrees of freedom analysis for each system that is evaluated