Answered step by step
Verified Expert Solution
Question
1 Approved Answer
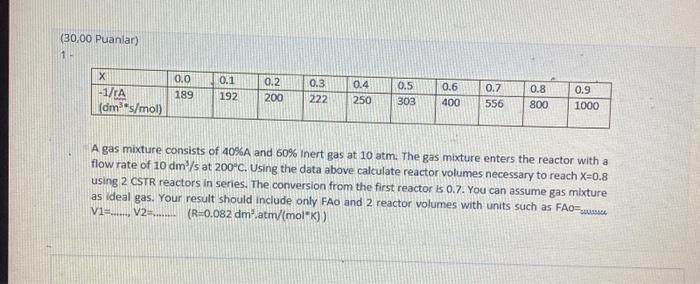
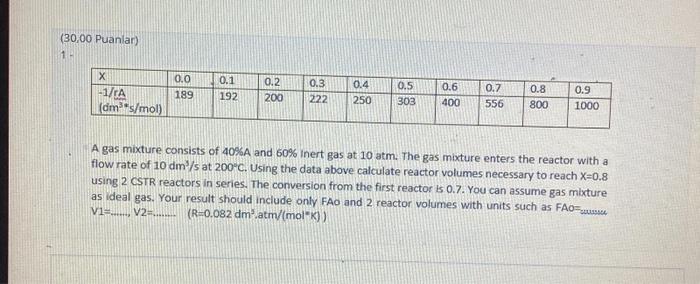
(30,00 Puanlar) X -1/rA (dm*s/mol) 0.0 189 0.1 192 0.2 200 0.3 222 0.4 250 0.5 303 0.6 400 0.7 556 0.8 800 0.9 1000
(30,00 Puanlar) X -1/rA (dm*s/mol) 0.0 189 0.1 192 0.2 200 0.3 222 0.4 250 0.5 303 0.6 400 0.7 556 0.8 800 0.9 1000 A gas mixture consists of 40%A and 60% Inert gas at 10 atm. The gas mixture enters the reactor with a flow rate of 10 dm/s at 200C. Using the data above calculate reactor volumes necessary to reach X=0.8 using 2 CSTR reactors in series. The conversion from the first reactor is 0.7. You can assume gas mixture as ideal gas. Your result should include only FAo and 2 reactor volumes with units such as FAO=.......... V1=......, V2=......... (R=0.082 dm.atm/(mol*K))
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


