Answered step by step
Verified Expert Solution
Question
1 Approved Answer
31 The number of protons, electrons and neutrons in aluminium ion Al+ is Protons A. 27 B. 13 C. ABCD 32 32. D. 13
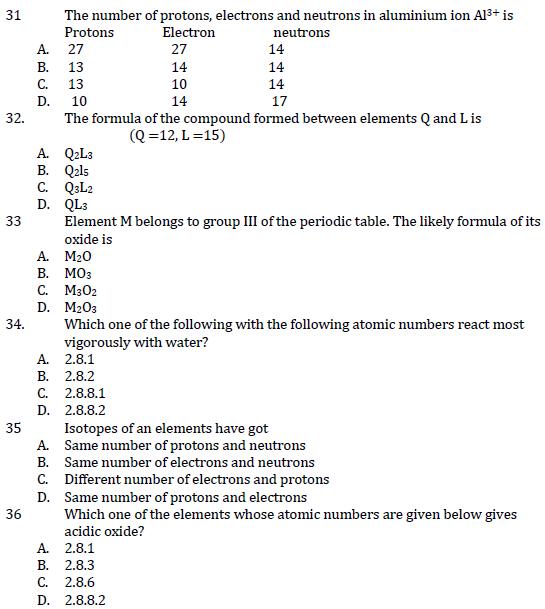
31 The number of protons, electrons and neutrons in aluminium ion Al+ is Protons A. 27 B. 13 C. ABCD 32 32. D. 13 10 Electron 27 neutrons 14 14 14 10 14 17 14 The formula of the compound formed between elements Q and Lis A. Q2L3 (Q=12, L=15) 33 B. Q2ls C. Q3L2 D. QL3 Element M belongs to group III of the periodic table. The likely formula of its oxide is M203 Which one of the following with the following atomic numbers react most vigorously with water? A. MO B. MO3 C. M302 D. 34. A. 2.8.1 B. 2.8.2 C. 2.8.8.1 D. 2.8.8.2 35 A. 36 Isotopes of an elements have got Same number of protons and neutrons B. Same number of electrons and neutrons C. Different number of electrons and protons D. Same number of protons and electrons Which one of the elements whose atomic numbers are given below gives acidic oxide? A. 2.8.1 B. 2.8.3 C. 2.8.6 D. 2.8.8.2
Step by Step Solution
★★★★★
3.36 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
The number of protons electrons and neutrons in the aluminum ion Al3 can be determined based on its atomic number The atomic number of aluminum Al is 13 which means it has 13 protons To find the numbe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


