Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3-10. Plot the polarization data shown below for an electrode of 1.13 cm of the metal M in an acid solution of unit activity
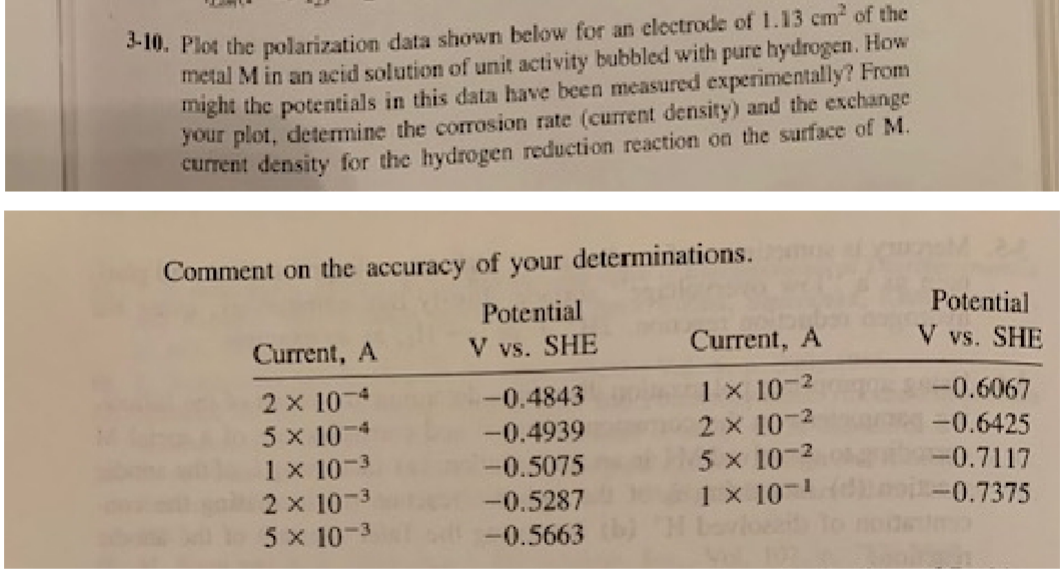
3-10. Plot the polarization data shown below for an electrode of 1.13 cm of the metal M in an acid solution of unit activity bubbled with pure hydrogen. How might the potentials in this data have been measured experimentally? From your plot, determine the corrosion rate (current density) and the exchange current density for the hydrogen reduction reaction on the surface of M. Comment on the accuracy of your determinations. Current, A Potential V vs. SHE Potential Current, A V vs. SHE 2 x 10 -0.4843 1 x 10-2 -0.6067 5 x 101 -0.4939 2 x 10-2 -0.6425 1 x 10-3 -0.5075 5 x 10-2 -0.7117 2 x 10-3 -0.5287 1 x 10-1 -0.7375. 5 x 10- -0.5663
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


