Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3.13 The reaction F + H H + HF is the rate-limiting elementary step in the overall reaction H + F 2 HF. This
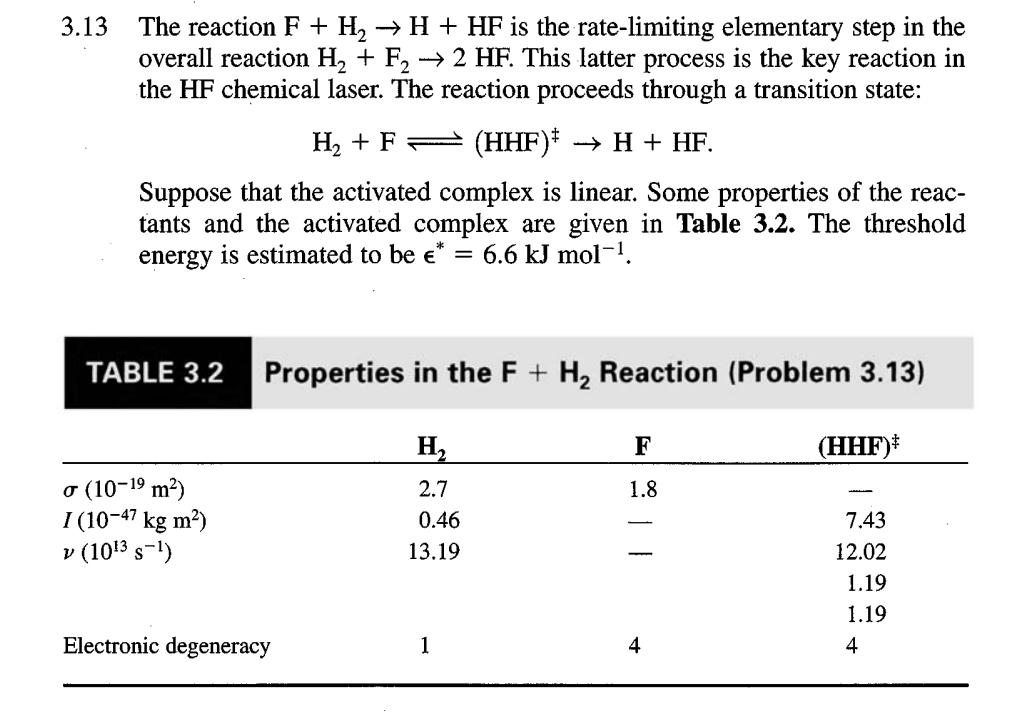

3.13 The reaction F + H H + HF is the rate-limiting elementary step in the overall reaction H + F 2 HF. This latter process is the key reaction in the HF chemical laser. The reaction proceeds through a transition state: H + F (HHF)* H + HF. Suppose that the activated complex is linear. Some properties of the reac- tants and the activated complex are given in Table 3.2. The threshold energy is estimated to be * = 6.6 kJ mol-. TABLE 3.2 (10-1 m) I (10-47 kg m) v (103 S-) Properties in the F + H Reaction (Problem 3.13) Electronic degeneracy H 2.7 0.46 13.19 1 F 1.8 4 (HHF)* 7.43 12.02 1.19 1.19 4 a. Use collision theory to calculate the preexponential factor and the rate constant for the reaction at 298 K. Compare your answer to the exper- imental result of A = 2 10 L mol- s-. b. Use activated complex theory to calculate the rate constant for the reac- tion at 298 K. Assume that the electronic degeneracy for F and for the activated complex is 4.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Part a Using Collision Theory To calculate the preexponential factor A and the rate constant k at 298 K using collision theory follow these steps 1 De...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


