electivity data for a process to produce acetic anhydride from acetone and acetic acid are given in the 1958 AIChE Student Contest Problem.* The

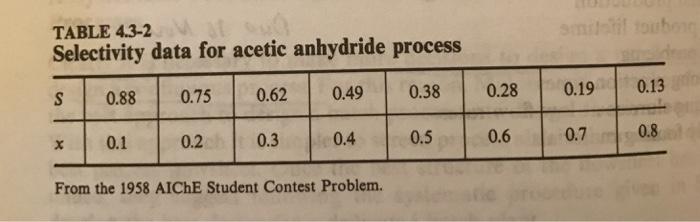
electivity data for a process to produce acetic anhydride from acetone and acetic acid are given in the 1958 AIChE Student Contest Problem.* The data are given in Table 4.3-2. The reactions of interest are Acetone - Ketene + CH 700C, 1 atm Ketene CO + }CH, 700C, 1 atm Ketene + Acetic Acid - Acetic Anhydride 80C, 1 atm mol ketene at reactor exit/mol acetone and the selectivity is defined as S converted. Develop a correlation for the data. Compare your results to the simple correlation S=1-x. Use your results to estimate the conversion corresponding to the maximum yield. smilslil toubong dene TABLE 4.3-2 Selectivity data for acetic anhydride process 0.88 0.75 0.62 0.49 0.38 0.28 0.19 0.13 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.1 From the 1958 AICHE Student Contest Problem.
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Given Selectivity data for Acetic anhydride process S mol ketene at reactor exit mo...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


