Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(35 points) You are an engineer at Heat Sinks Co assigned to a team designing a heat sink to cool an LED light panel. The
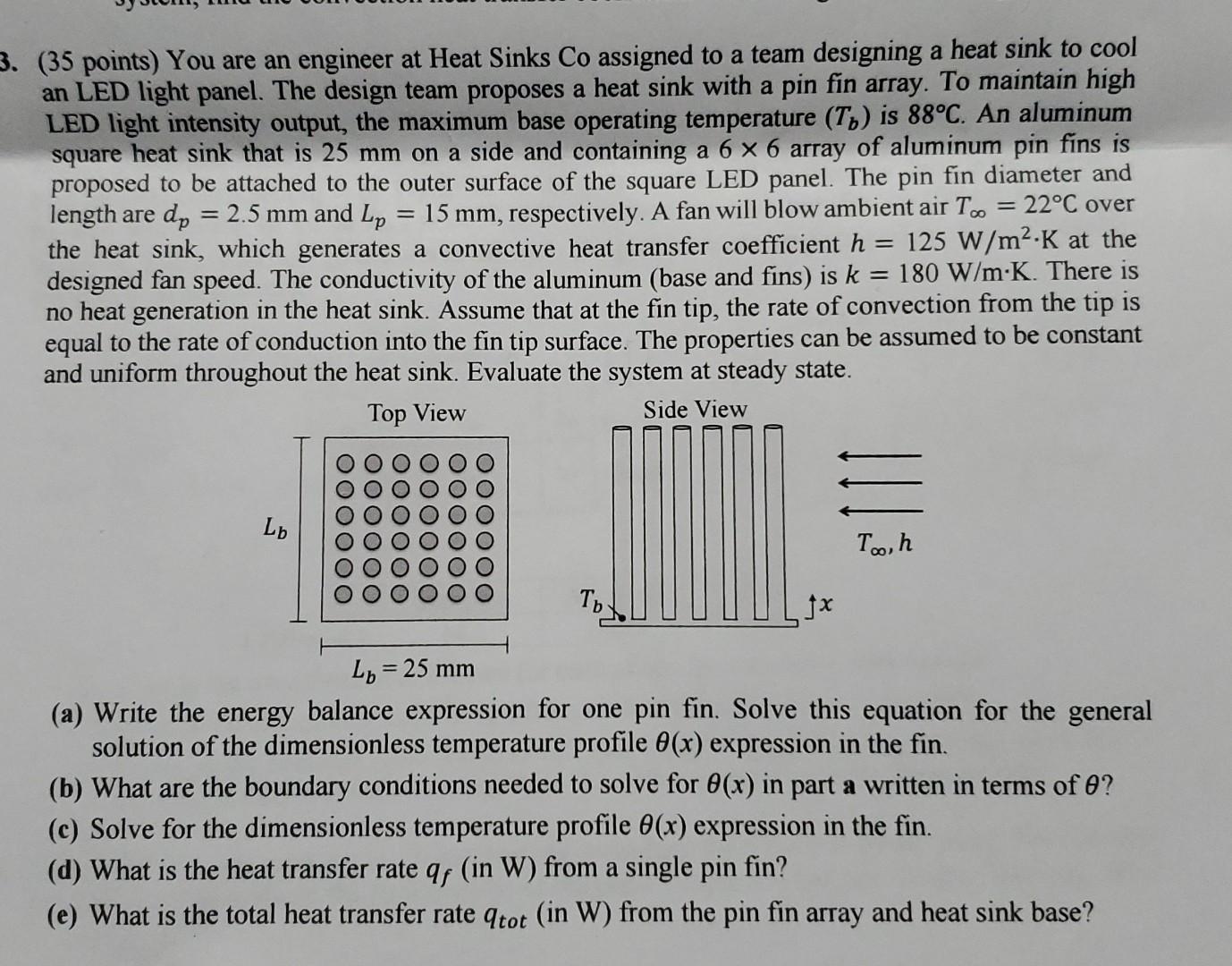
(35 points) You are an engineer at Heat Sinks Co assigned to a team designing a heat sink to cool an LED light panel. The design team proposes a heat sink with a pin fin array. To maintain high LED light intensity output, the maximum base operating temperature (Tb) is 88C. An aluminum square heat sink that is 25mm on a side and containing a 66 array of aluminum pin fins is proposed to be attached to the outer surface of the square LED panel. The pin fin diameter and length are dp=2.5mm and Lp=15mm, respectively. A fan will blow ambient air T=22C over the heat sink, which generates a convective heat transfer coefficient h=125W/m2K at the designed fan speed. The conductivity of the aluminum (base and fins) is k=180W/mK. There is no heat generation in the heat sink. Assume that at the fin tip, the rate of convection from the tip is equal to the rate of conduction into the fin tip surface. The properties can be assumed to be constant and uniform throughout the heat sink. Evaluate the system at steady state. (a) Write the energy balance expression for one pin fin. Solve this equation for the general solution of the dimensionless temperature profile (x) expression in the fin. (b) What are the boundary conditions needed to solve for (x) in part a written in terms of ? (c) Solve for the dimensionless temperature profile (x) expression in the fin. (d) What is the heat transfer rate qf (in W) from a single pin fin? (e) What is the total heat transfer rate qtot (in W) from the pin fin array and heat sink base
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


