Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(35 pts.) The reversible gas-phase reaction (forward and reverse reactions are elementary), AB is processed in an adiabatic CSTR. The inlet consists of pure
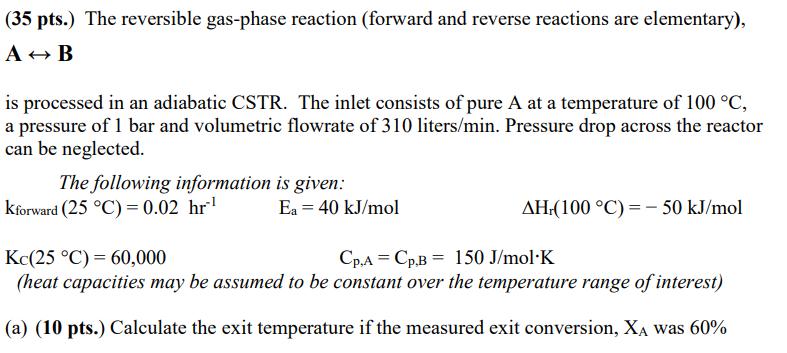
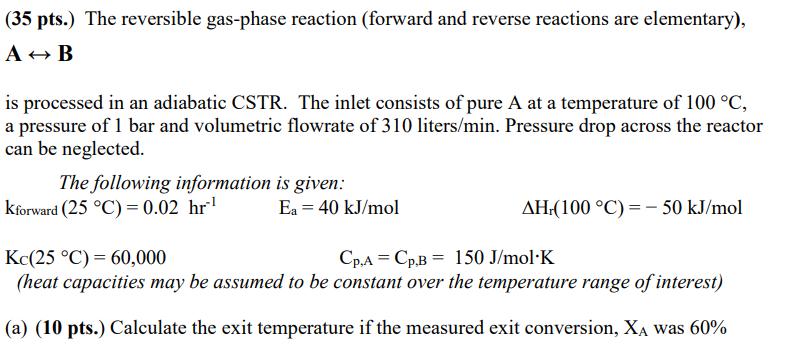

(35 pts.) The reversible gas-phase reaction (forward and reverse reactions are elementary), AB is processed in an adiabatic CSTR. The inlet consists of pure A at a temperature of 100 C, a pressure of 1 bar and volumetric flowrate of 310 liters/min. Pressure drop across the reactor can be neglected. The following information is given: Kforward (25 C) = 0.02 hr Kc(25 C) 60,000 Ea = 40 kJ/mol AH.(100 C) - 50 kJ/mol Cp.A Cp.B 150 J/mol K (heat capacities may be assumed to be constant over the temperature range of interest) (a) (10 pts.) Calculate the exit temperature if the measured exit conversion, XA was 60% (35 pts.) The reversible gas-phase reaction (forward and reverse reactions are elementary), AB is processed in an adiabatic CSTR. The inlet consists of pure A at a temperature of 100 C, a pressure of 1 bar and volumetric flowrate of 310 liters/min. Pressure drop across the reactor can be neglected. The following information is given: Kforward (25 C) = 0.02 hr Kc(25 C) 60,000 Ea = 40 kJ/mol AH.(100 C) - 50 kJ/mol Cp.A Cp.B 150 J/mol K (heat capacities may be assumed to be constant over the temperature range of interest) (a) (10 pts.) Calculate the exit temperature if the measured exit conversion, XA was 60% (c) (10 pts.) Calculate the volume of the adiabatic CSTR if the measured exit conversion was 60%.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


