Answered step by step
Verified Expert Solution
Question
1 Approved Answer
39. Ethylene oxide as a vapor and water as liquid, both at 25C and 101.33kPa, react to form a liquid solution containing ethylene glycol (1,2-ethanediol)
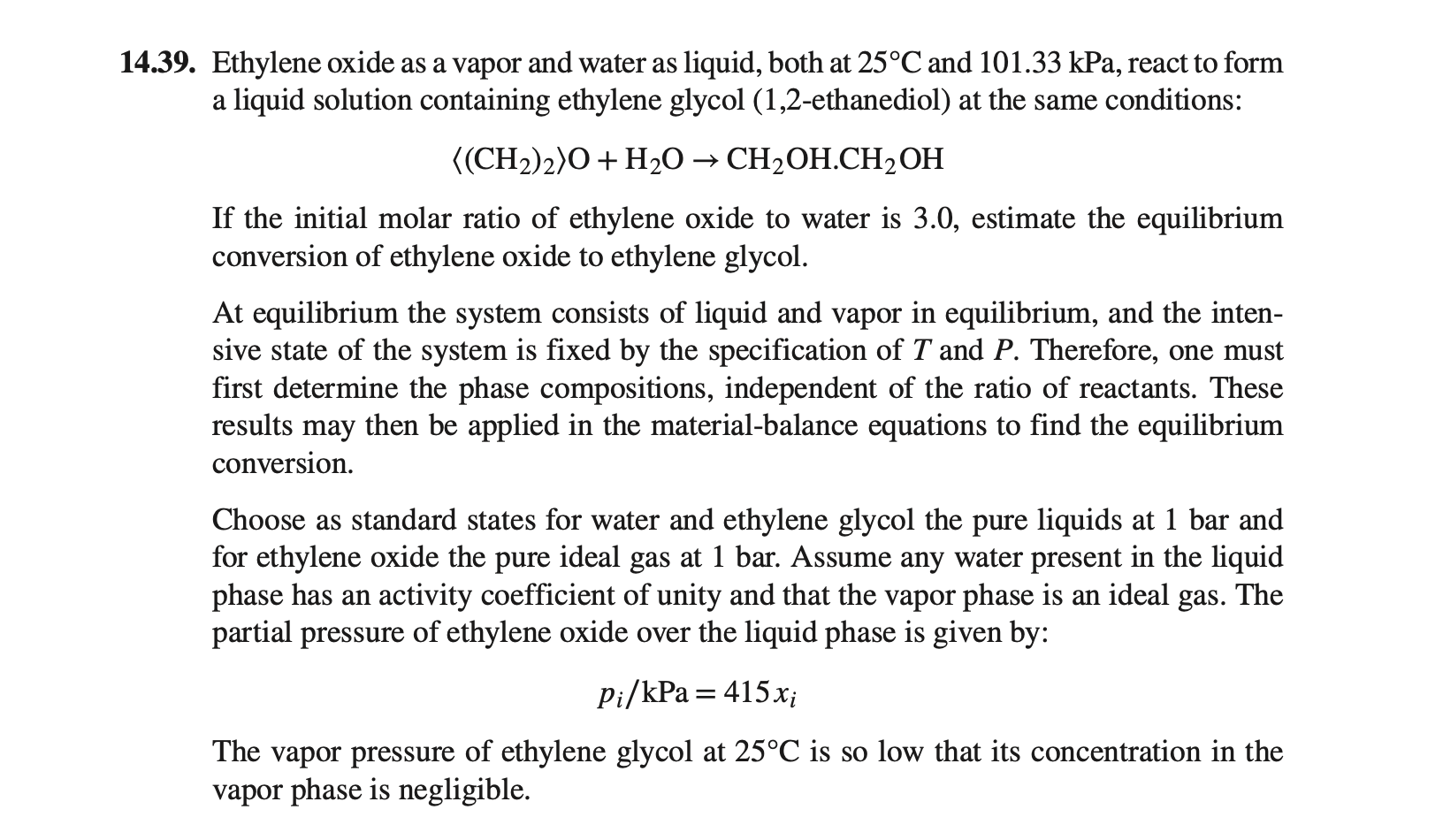 39. Ethylene oxide as a vapor and water as liquid, both at 25C and 101.33kPa, react to form a liquid solution containing ethylene glycol (1,2-ethanediol) at the same conditions: (CH2)2O+H2OCH2OH.CH2OH If the initial molar ratio of ethylene oxide to water is 3.0 , estimate the equilibrium conversion of ethylene oxide to ethylene glycol. At equilibrium the system consists of liquid and vapor in equilibrium, and the intensive state of the system is fixed by the specification of T and P. Therefore, one must first determine the phase compositions, independent of the ratio of reactants. These results may then be applied in the material-balance equations to find the equilibrium conversion. Choose as standard states for water and ethylene glycol the pure liquids at 1 bar and for ethylene oxide the pure ideal gas at 1 bar. Assume any water present in the liquid phase has an activity coefficient of unity and that the vapor phase is an ideal gas. The partial pressure of ethylene oxide over the liquid phase is given by: pi/kPa=415xi The vapor pressure of ethylene glycol at 25C is so low that its concentration in the vapor phase is negligible
39. Ethylene oxide as a vapor and water as liquid, both at 25C and 101.33kPa, react to form a liquid solution containing ethylene glycol (1,2-ethanediol) at the same conditions: (CH2)2O+H2OCH2OH.CH2OH If the initial molar ratio of ethylene oxide to water is 3.0 , estimate the equilibrium conversion of ethylene oxide to ethylene glycol. At equilibrium the system consists of liquid and vapor in equilibrium, and the intensive state of the system is fixed by the specification of T and P. Therefore, one must first determine the phase compositions, independent of the ratio of reactants. These results may then be applied in the material-balance equations to find the equilibrium conversion. Choose as standard states for water and ethylene glycol the pure liquids at 1 bar and for ethylene oxide the pure ideal gas at 1 bar. Assume any water present in the liquid phase has an activity coefficient of unity and that the vapor phase is an ideal gas. The partial pressure of ethylene oxide over the liquid phase is given by: pi/kPa=415xi The vapor pressure of ethylene glycol at 25C is so low that its concentration in the vapor phase is negligible Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


