Answered step by step
Verified Expert Solution
Question
1 Approved Answer
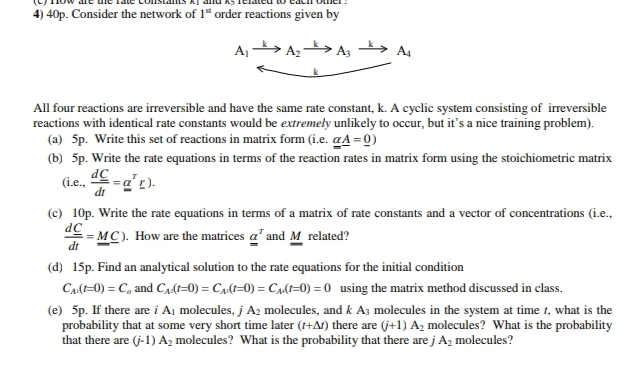
4 0 p . Consider the network of 1 s t order reactions given by All four reactions are irreversible and have the same rate
Consider the network of order reactions given by
All four reactions are irreversible and have the same rate constant, A cyclic system consisting of irreversible
reactions with identical rate constants would be extremely unlikely to occur, but it's a nice training problem
a p Write this set of reactions in matrix form ie
b p Write the rate equations in terms of the reaction rates in matrix form using the stoichiometric matrix
ie
c p Write the rate equations in terms of a matrix of rate constants and a vector of concentrations ie
How are the matrices and related?
d p Find an analytical solution to the rate equations for the initial condition
and using the matrix method discussed in class.
e If there are molecules, molecules, and molecules in the system at time what is the
probability that at some very short time later there are molecules? What is the probability
that there are molecules? What is the probability that there are molecules?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


