Answered step by step
Verified Expert Solution
Question
1 Approved Answer
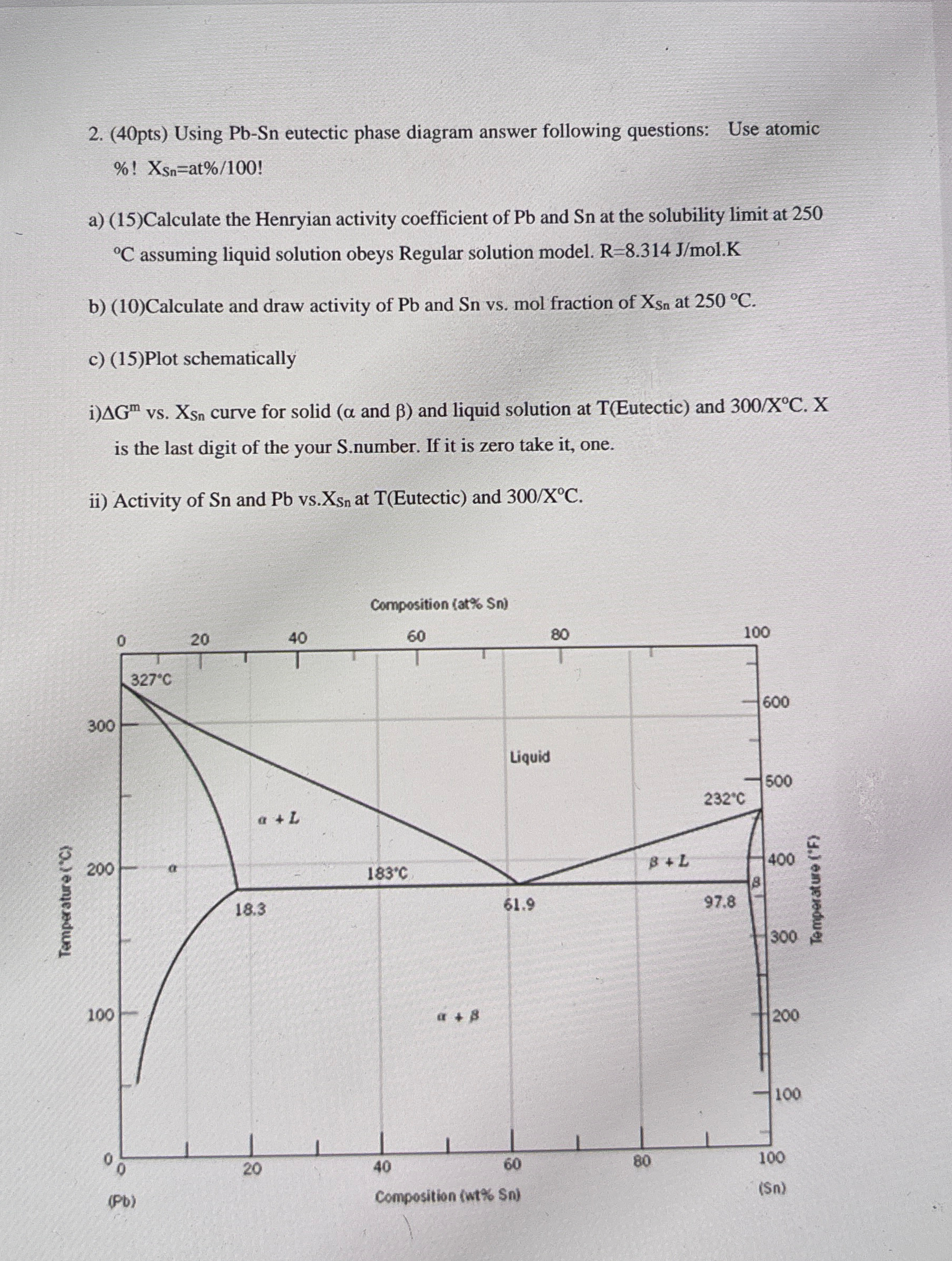
( 4 0 pts ) Using Pb - Sn eutectic phase diagram answer following questions: Use atomic % ! x S n = a t
pts Using PbSn eutectic phase diagram answer following questions: Use atomic
aCalculate the Henryian activity coefficient of and at the solubility limit at assuming liquid solution obeys Regular solution model.
bCalculate and draw activity of and vs mol fraction of at
cPlot schematically
i vs curve for solid and and liquid solution at Eutectic and is the last digit of the your Snumber. If it is zero take it one.
ii Activity of and vs at Eutectic and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


