Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4 1 point Which Lewis structure would the phosphide ion have in calcium phosphide? X O |:X: [:X:] [X] 3- 5 1 point The

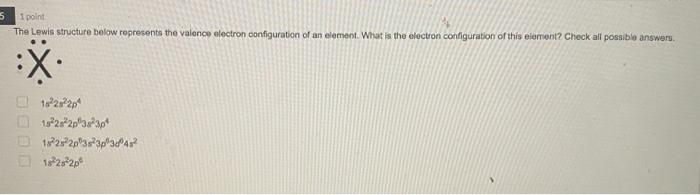
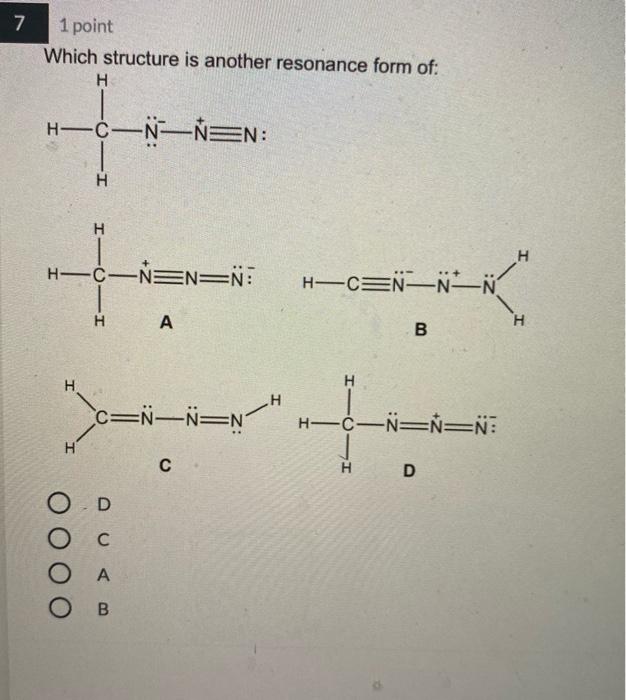
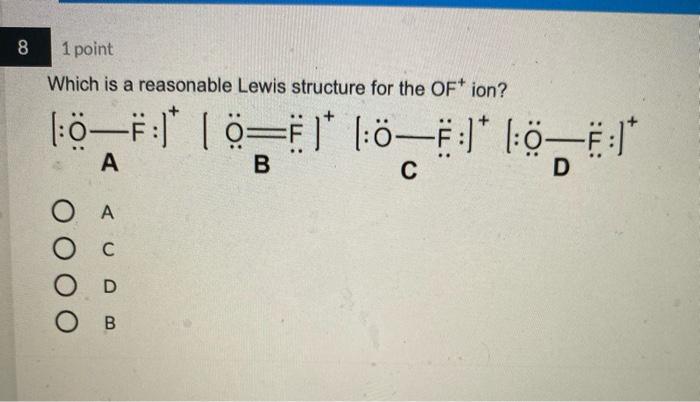

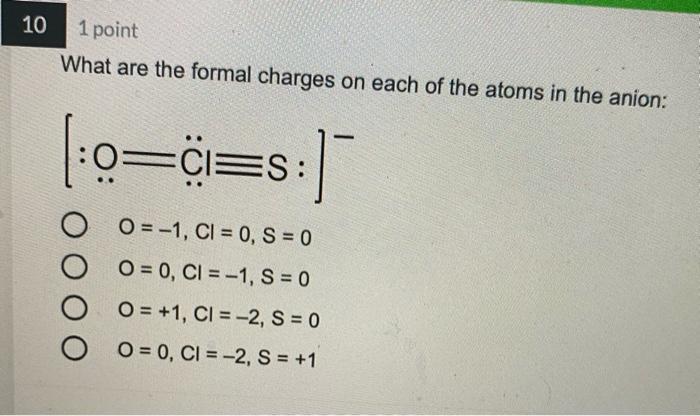
4 1 point Which Lewis structure would the phosphide ion have in calcium phosphide? X O |:X: [:X:] [X] 3- 5 1 point The Lewis structure below represents the valence electron configuration of an element. What is the electron configuration of this element? Check all possible answers. :X. 18282p 1822s 2p 3s 3p 1s 2s 2p 3s 3p 3d 4 182s2p 7 1 point Which structure is another resonance form of: H HNN=N: H H-C-NEN=N: H A H H -H N=NN=0 O D C OB C H-CNN N B H HCN=N=N: D H H 8 1 point Which is a reasonable Lewis structure for the OF* ion? |:0F:)* | Q=F ]* |:0F:]* [:0F:]* A D 0 000 ACDB O c 9 1 point In the Lewis structure of the nitrite anion, NO, if all atoms obey the octet rule, there must be: O2 N=0 bonds 2 N-O bonds 1 N-O bond and 1 NEO bond 1 N-O bond and 1 N=O bond 10 1 point What are the formal charges on each of the atoms in the anion: |:0=c=s: s:] O O=-1, CI = 0, S = 0 O=0, CI = -1, S = 0 O=+1, CI = -2, S = 0 O=0, CI = -2, S = +1
Step by Step Solution
★★★★★
3.31 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1
4 which lewis stuetuu would the phosphide ion have in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


