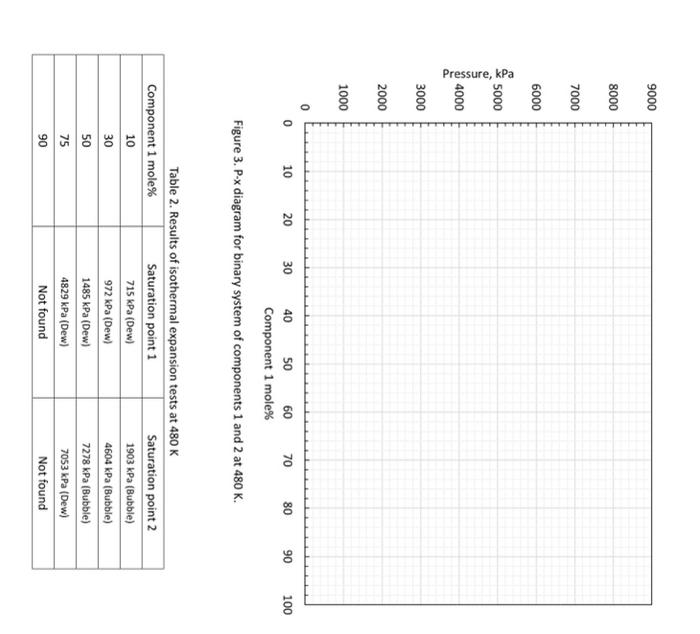
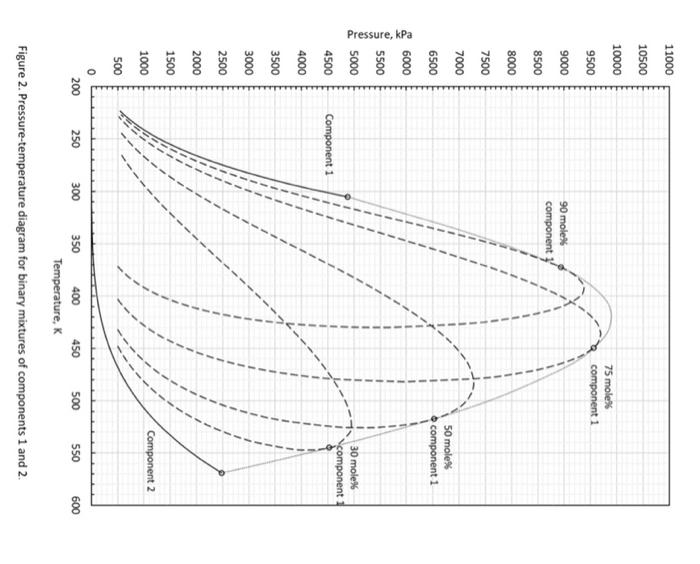
4. (35 points) A student performed isothermal expansion tests for five mixtures of component 1 and component 2 at 480 Kelvin (so, they are binary mixtures). The PT projection for this binary system is given in the figure below. a. (5 points) From Figure 2, which is more volatile, component 1 or 2 ? Why? b. (5 points) From Figure 2, what is the vapor pressure of component 2 at 480K ? c. (5 points) From Figure 2, do you expect that a certain mixture of components 1 and 2 exhibits a critical point at 480K ? If so, at what pressure will the critical point occur (for a certain mixture of components 1 and 2 )? d. (5 points) Table 2 shows the data obtained by the isothermal expansion tests. In Figure 3, carefully draw a two-phase envelope for this binary system at 480K using the data provided. In Figure 3, clearly indicate the vapor pressure that you answered for part b, and the critical point that you answered for part c. e. (5 points) In part c, you found a critical pressure at 480K for a certain mixture of components 1 and 2 . Now with your answer to part d, what is the approximate composition that will exhibit the critical point at 480K ? Write down the composition, and also indicate your critical point in Figure 3 clearly. f. (5 points) Based on Figure 3, what is the maximum concentration of component 1 (mol\%) up to which you can keep a single liquid phase at 4,000kPa at 480K ? g. (5 points) Based on Figure 3, what is the equilibrium liquid composition when the overall composition is 50 mol\% component 1 and 50 mol\% component 2 at 6,000kPa at 480K ? Figure 3. P-x diagram for binary system of components 1 and 2 at 480K. Table 2. Results of isothermal expansion tests at 480K 4. (35 points) A student performed isothermal expansion tests for five mixtures of component 1 and component 2 at 480 Kelvin (so, they are binary mixtures). The PT projection for this binary system is given in the figure below. a. (5 points) From Figure 2, which is more volatile, component 1 or 2 ? Why? b. (5 points) From Figure 2, what is the vapor pressure of component 2 at 480K ? c. (5 points) From Figure 2, do you expect that a certain mixture of components 1 and 2 exhibits a critical point at 480K ? If so, at what pressure will the critical point occur (for a certain mixture of components 1 and 2 )? d. (5 points) Table 2 shows the data obtained by the isothermal expansion tests. In Figure 3, carefully draw a two-phase envelope for this binary system at 480K using the data provided. In Figure 3, clearly indicate the vapor pressure that you answered for part b, and the critical point that you answered for part c. e. (5 points) In part c, you found a critical pressure at 480K for a certain mixture of components 1 and 2 . Now with your answer to part d, what is the approximate composition that will exhibit the critical point at 480K ? Write down the composition, and also indicate your critical point in Figure 3 clearly. f. (5 points) Based on Figure 3, what is the maximum concentration of component 1 (mol\%) up to which you can keep a single liquid phase at 4,000kPa at 480K ? g. (5 points) Based on Figure 3, what is the equilibrium liquid composition when the overall composition is 50 mol\% component 1 and 50 mol\% component 2 at 6,000kPa at 480K ? Figure 3. P-x diagram for binary system of components 1 and 2 at 480K. Table 2. Results of isothermal expansion tests at 480K