Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. In a Bradford experiment, you make four dilutions (1:5, 1:10; 1:100 and 1:1000) of an unknown stock protein. Using a BSA control, you
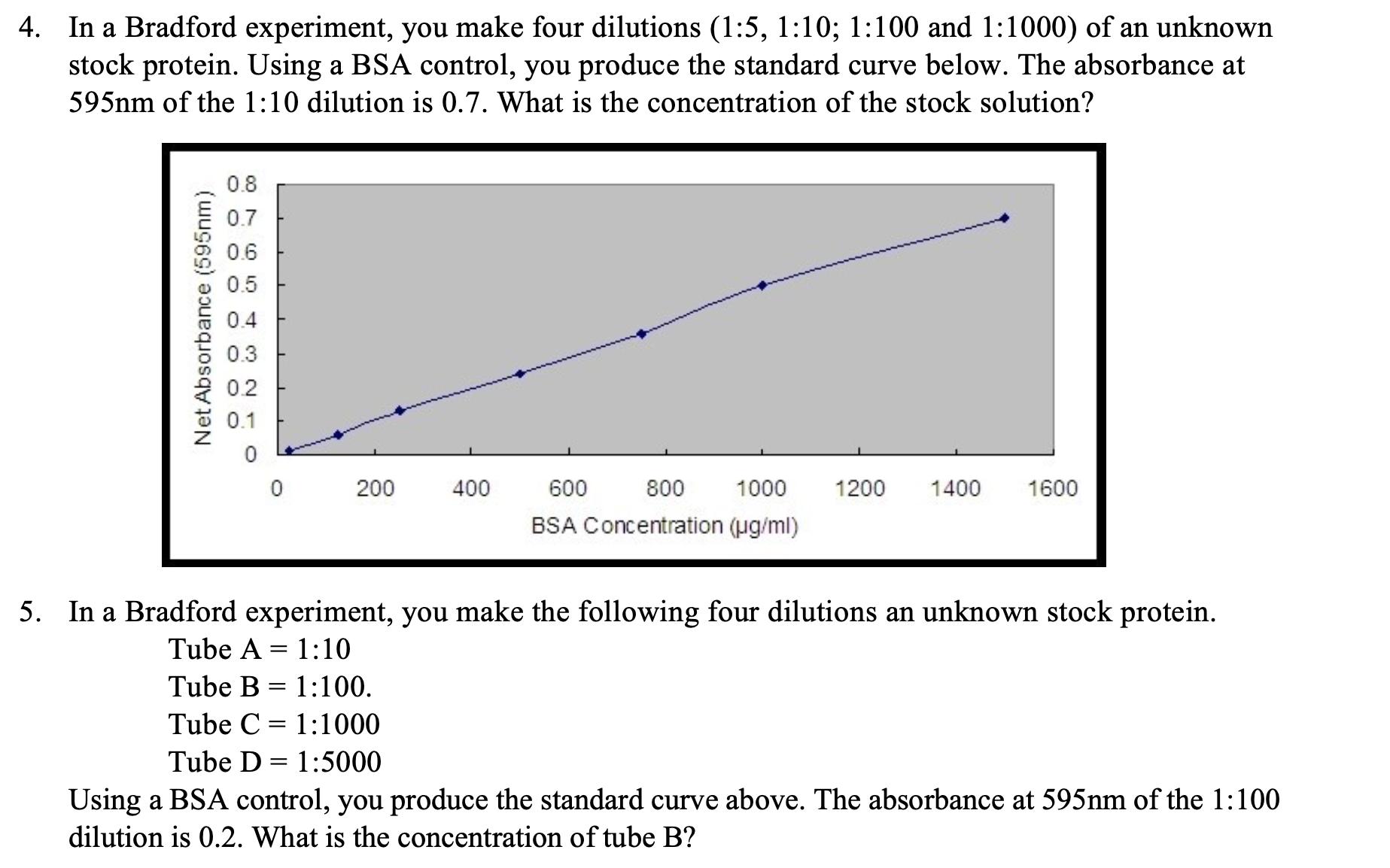
4. In a Bradford experiment, you make four dilutions (1:5, 1:10; 1:100 and 1:1000) of an unknown stock protein. Using a BSA control, you produce the standard curve below. The absorbance at 595nm of the 1:10 dilution is 0.7. What is the concentration of the stock solution? 0.8 0.7 0.6 0.5 0.4 Net Absorbance (595nm) 0.3 0.2 0.1 0 200 400 600 1000 BSA Concentration (g/ml) 800 1200 1400 1600 5. In a Bradford experiment, you make the following four dilutions an unknown stock protein. Tube A1:10 Tube B 1:100. Tube C=1:1000 Tube D 1:5000 = Using a BSA control, you produce the standard curve above. The absorbance at 595nm of the 1:100 dilution is 0.2. What is the concentration of tube B?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
4 we have a standard curve with us and we can use it to find the concentration of an ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


