Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. In the dilute limit, there are many more accessible states than particles. This implies that the average occupation of any state should be

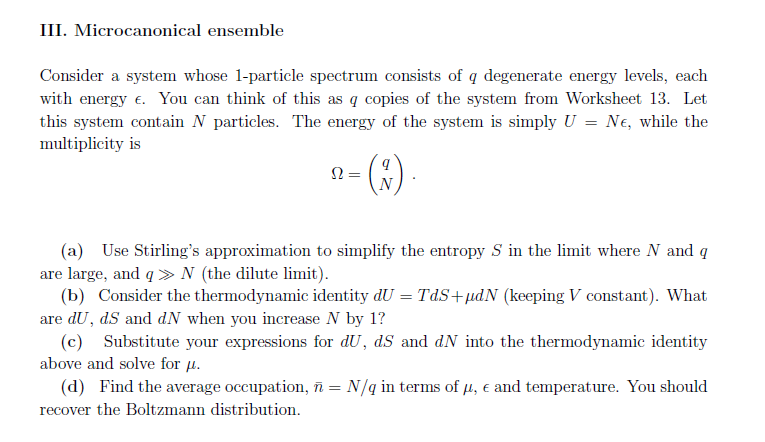
4. In the dilute limit, there are many more accessible states than particles. This implies that the average occupation of any state should be very small. We call the distribution n(, T, ) in this limit the Boltzmann distribution. In this question, will can examine it in detail in all three ensembles inctroduced in the course. I. Grand canonical ensemble (a) Consider the Bose-Einstein distribution. Fixing e and T, for what chemical potential is BE very small? (b) For the situation in the previous part, show that BE is approximately e-(E-). (c) Repeat parts (a) and (b) above for the Fermi-Dirac distribution. II. Canonical ensemble Consider a system whose 1-particle spectrum contains a level with energy (and a whole bunch other levels too). Let Z be the partition function for one particle in this system and == ZN (Z1) be the partition function for N identical particles in the dilute limit. (a) Find the chemical potential in the limit of large N (use Stirling's approximation), in terms of Z and other quantities. (b) Use your answer to part (a) to find Z in terms of the parameters , and N. (c) If the system contains just one particle, the probability of it being in our selected state with energy is e-/Z. Argue (using the fact that the system is dilute) that (the average number of particles in our selected state) is Ne-BE/Z1. (d) Substitute your result from (b) into (c) and recover the Boltzmann distribution. III. Microcanonical ensemble Consider a system whose 1-particle spectrum consists of q degenerate energy levels, each with energy . You can think of this as q copies of the system from Worksheet 13. Let this system contain N particles. The energy of the system is simply U = Ne, while the multiplicity is = (x) (a) Use Stirling's approximation to simplify the entropy S in the limit where N and q are large, and q >>> N (the dilute limit). (b) Consider the thermodynamic identity dU = TdS+dN (keeping V constant). What are du, dS and dN when you increase N by 1? (c) Substitute your expressions for dU, dS and dN into the thermodynamic identity above and solve for . (d) Find the average occupation, n = N/q in terms of , e and temperature. You should recover the Boltzmann distribution.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


