Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. In the gold foil experiment (Au; Z=79), alpha particles with Z=2, m = 4.0000 a.m.u have an initial kinetic energy of 7.9384 MeV.
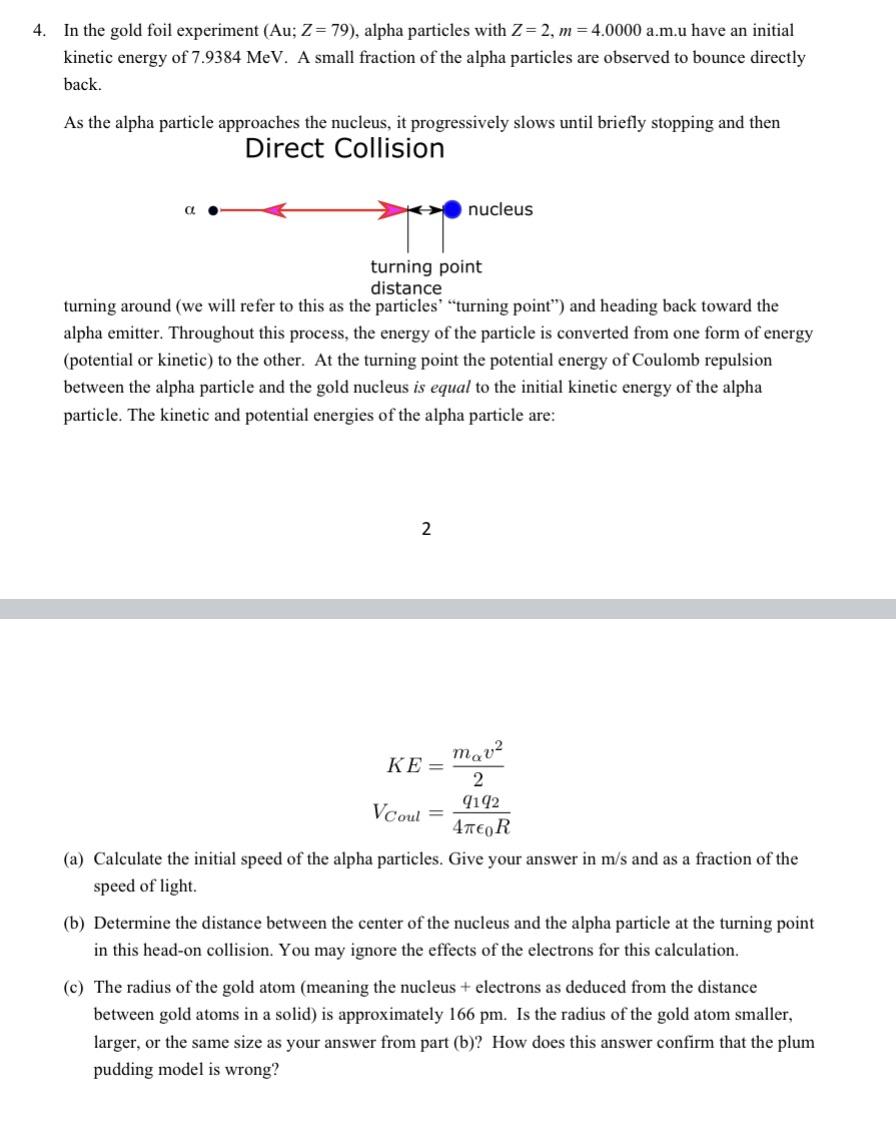
4. In the gold foil experiment (Au; Z=79), alpha particles with Z=2, m = 4.0000 a.m.u have an initial kinetic energy of 7.9384 MeV. A small fraction of the alpha particles are observed to bounce directly back. As the alpha particle approaches the nucleus, it progressively slows until briefly stopping and then Direct Collision a nucleus turning point distance turning around (we will refer to this as the particles' "turning point") and heading back toward the alpha emitter. Throughout this process, the energy of the particle is converted from one form of energy (potential or kinetic) to the other. At the turning point the potential energy of Coulomb repulsion between the alpha particle and the gold nucleus is equal to the initial kinetic energy of the alpha particle. The kinetic and potential energies of the alpha particle are: 2 mav2 KE= 2 9192 VCoul = (a) Calculate the initial speed of the alpha particles. Give your answer in m/s and as a fraction of the speed of light. (b) Determine the distance between the center of the nucleus and the alpha particle at the turning point in this head-on collision. You may ignore the effects of the electrons for this calculation. (c) The radius of the gold atom (meaning the nucleus + electrons as deduced from the distance between gold atoms in a solid) is approximately 166 pm. Is the radius of the gold atom smaller, larger, or the same size as your answer from part (b)? How does this answer confirm that the plum pudding model is wrong?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


