Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Methane burns in air with an equivalence ratio of =0.85, determine the composition of the products: What are the Mass Fractions of each

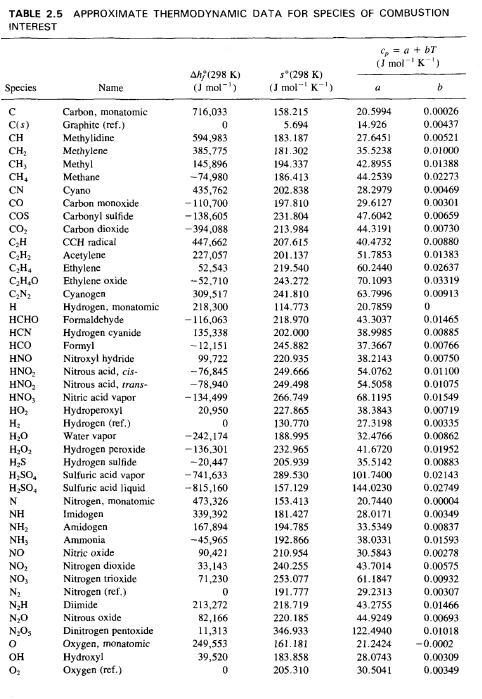
4. Methane burns in air with an equivalence ratio of =0.85, determine the composition of the products: What are the Mass Fractions of each species in the Products? TABLE 2.5 APPROXIMATE THERMODYNAMIC DATA FOR SPECIES OF COMBUSTION INTEREST G = a + bT (J mol K) Species Name Ah(298 K) (J mol) s(298 K) (J mol K) a b Carbon, monatomic 716,033 158.215 20.5994 0.00026 C(s) Graphite (ref.) 0 5.694 14.926 0.00437 CH Methylidine 594,983 183.187 27.6451 0.00521 CH Methylene 385,775 181.302 35.5238 0.01000 CH, Methyl 145,896 194.337 42.8955 0.01388 CH Methane -74,980 186.413 44.2539 0.02273 CN Cyano 435,762 202.838 28.2979 0.00469 Carbon monoxide -110,700 197.810 29.6127 0.00301 COS Carbonyl sulfide -138,605 231.804 47.6042 0.00659 Carbon dioxide -394,088 213.984 44.3191 0.00730 CH CCH radical 447,662 207.615 40.4732 0.00880 CH Acetylene 227,057 201.137 51.7853 0.01383 CH4 Ethylene 52,543 219.540 60.2440 0.02637 CHO Ethylene oxide -52,710 243.272 70.1093 0.033191 CN Cyanogen 309,517 241.810 63.7996 0.00913 H Hydrogen, monatomic 218,300 114.773 20.7859 0 HCHO Formaldehyde -116,063 218.970 43.3037 0.01465 HCN Hydrogen cyanide 135,338 202.000 38.9985 0.00885 HCO Formyl -12,151 245.882 37.3667 0.00766 HNO Nitroxyl hydride 99,722 220.935 38.2143 0.00750 , Nitrous acid, cis- -76,845 249.666 54.0762 0.01100 , Nitrous acid, trans- -78,940 249.498 54.5058 0.01075 HNO, Nitric acid vapor -134,499 266.749 68.1195 0.01549 HO Hydroperoxyl 20,950 227.865 38.3843 0.00719 H Hydrogen (ref.) 0 130.770 27.3198 0.00335 HO Water vapor -242,174 188.995 32.4766 0.00862 HO Hydrogen peroxide -136,301 232.965 41.6720 0.01952 HS Hydrogen sulfide -20,447 205.939 35.5142 0.00883 HSO Sulfuric acid vapor -741,633 289.530 101.7400 0.02143 HSO Sulfuric acid liquid -815,160 157.129 144.0230 0.02749 N Nitrogen, monatomic 473,326 153.413 20.7440 0.00004 NH Imidogen 339,392 181.427 28.0171 0.00349 NH Amidogen 167,894 194.785 33.5349 0.00837 NH, Ammonia -45,965 192.866 38.0331 0.01593 NO Nitric oxide 90,421 210.954 30.5843 0.00278 NO Nitrogen dioxide 33,143 240.255 43.7014 0.00575 NO3 Nitrogen trioxide 71,230 253.077 61.1847 0.00932 N Nitrogen (ref.) 0 191.777 29.2313 0.00307 NH Diimide 213,272 218.719 43.2755 0.01466 NO Nitrous oxide 82,166 220.185 44.9249 0.00693 N.Os Dinitrogen pentoxide 11,313 346.933 122.4940 0.01018 Oxygen, monatomic 249,553 161.181 21.2424 -0.0002 OH Hydroxyl 39,520 183.858 28.0743 0.00309 Oxygen (ref.) 0 205.310 30.5041 0.00349
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


