Question
4. Nitrogen is unusual in that it can be many forms in the environment, with oxidation states -III, III and V common in water

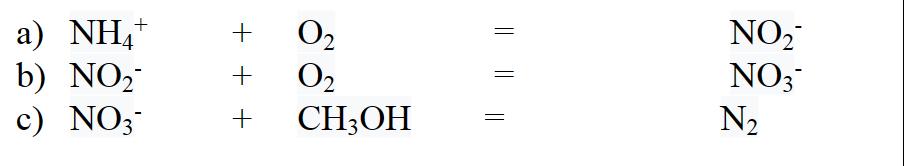
4. Nitrogen is unusual in that it can be many forms in the environment, with oxidation states -III, III and V common in water and N or N(0) relatively unreactive with its triple bond. To remove nitrogen pollution (as a nutrient), ammonium (N(-III)) is oxidized to nitrite (N(III)) and then further oxidized to nitrate (N(V)) in a process called nitrification, which is then reduced to N in a process called denitrification. Write balanced redox reactions for the following steps (where you are given both the oxidant and the reductant). a) NH4+ b) NO c) NO3- +++ 0 0 CH3OH NO NO3 N
Step by Step Solution
3.61 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The complete balanced redox ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Management A Practical Introduction
Authors: Angelo Kinicki, Brian Williams
5th edition
978-1111821227, 9781133190363, 1111821224, 1133190367, 978-0078112713
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



