Answered step by step
Verified Expert Solution
Question
1 Approved Answer
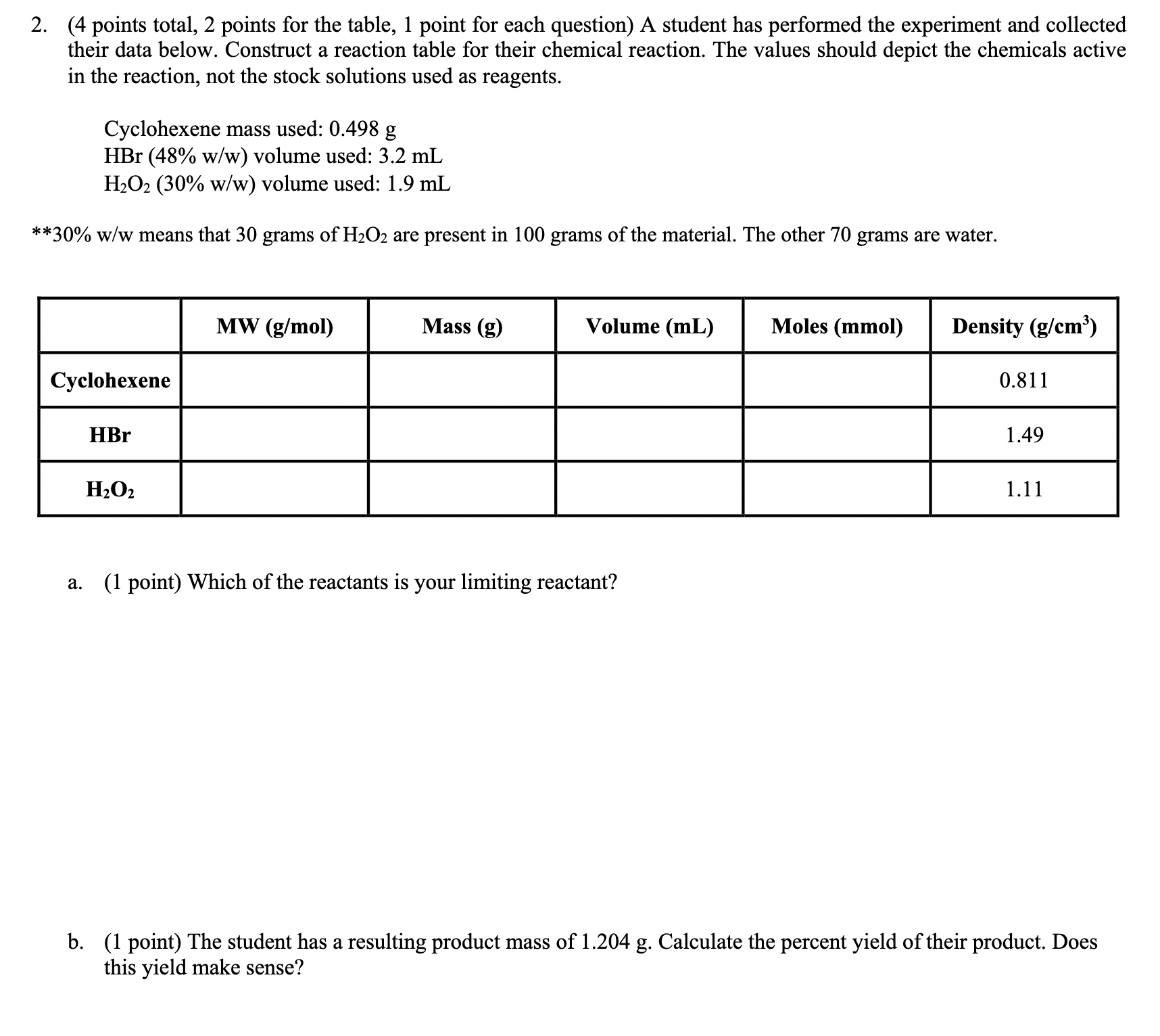
( 4 points total, 2 points for the table, 1 point for each question ) A student has performed the experiment and collected their data
points total, points for the table, point for each question A student has performed the experiment and collected
their data below. Construct a reaction table for their chemical reaction. The values should depict the chemicals active
in the reaction, not the stock solutions used as reagents.
Cyclohexene mass used:
volume used:
volume used:
ww means that grams of are present in grams of the material. The other grams are water.
a point Which of the reactants is your limiting reactant?
b point The student has a resulting product mass of Calculate the percent yield of their product. Does
this yield make sense?
The equation is HBRHOOH BRHO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


