Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4 Tetrachlorosilane (SiCl4) gas is reacted with hydrogen gas (H2) to produce electronic-grade polycrystalline silicon at 800C and 1.5 x 105 Pa according to
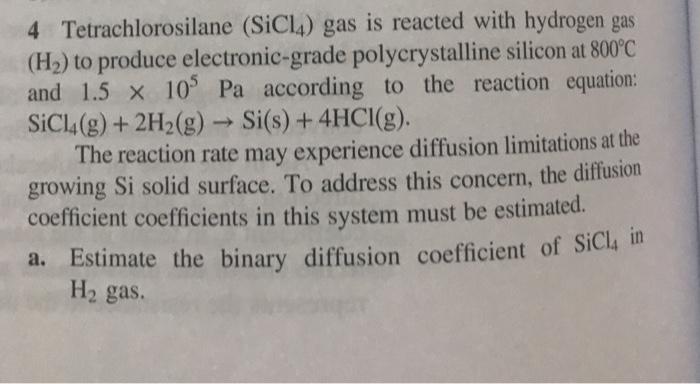
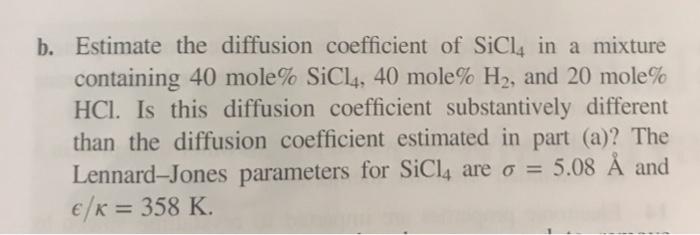
4 Tetrachlorosilane (SiCl4) gas is reacted with hydrogen gas (H2) to produce electronic-grade polycrystalline silicon at 800C and 1.5 x 105 Pa according to the reaction equation: SiCl4(g)+2H2(g) Si(s) + 4HCl(g). -> The reaction rate may experience diffusion limitations at the growing Si solid surface. To address this concern, the diffusion coefficient coefficients in this system must be estimated. a. Estimate the binary diffusion coefficient of SiCl4 in H gas. b. Estimate the diffusion coefficient of SiCl4 in a mixture containing 40 mole% SiCl4, 40 mole% H2, and 20 mole% HCl. Is this diffusion coefficient substantively different than the diffusion coefficient estimated in part (a)? The Lennard-Jones parameters for SiCl4 are = 5.08 and /K = 358 K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


