Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. The attached figure is a polarization curve from an experiment with K3 [Fe(CN)6]/K4[Fe(CN)6]. (a) Please determine the equilibrium potential and limiting current of the
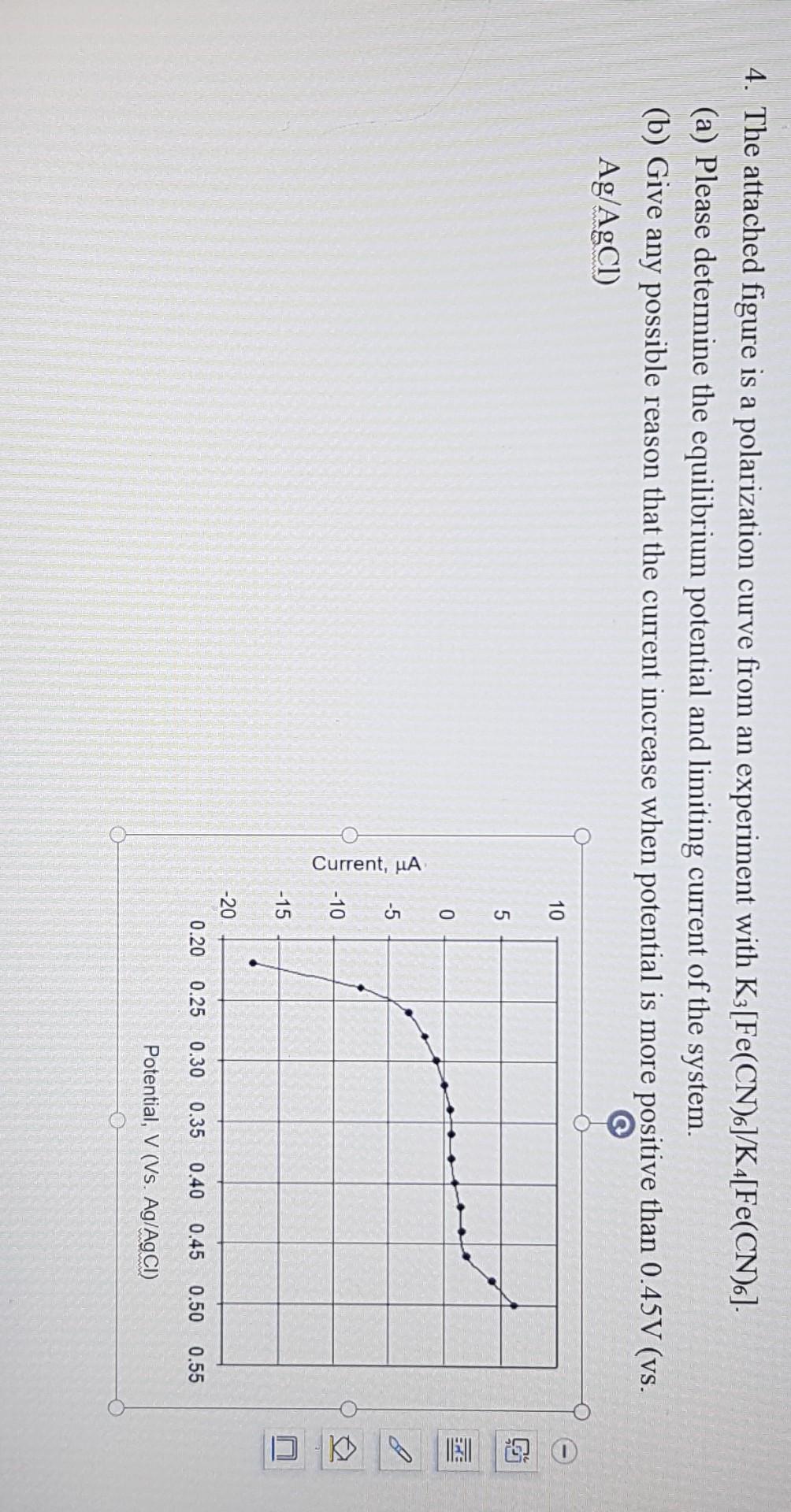
4. The attached figure is a polarization curve from an experiment with K3 [Fe(CN)6]/K4[Fe(CN)6]. (a) Please determine the equilibrium potential and limiting current of the system. (b) Give any possible reason that the current increase when potential is more positive than 0.45V (vs. Ag/AgCl) - 10 5 BU 0 -5 I Current, uA -10 -15 -20 0.20 0.25 0.30 0.35 0.40 0.45 0.50 0.55 Potential, V (Vs. Ag/AgCl)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


