Answered step by step
Verified Expert Solution
Question
1 Approved Answer
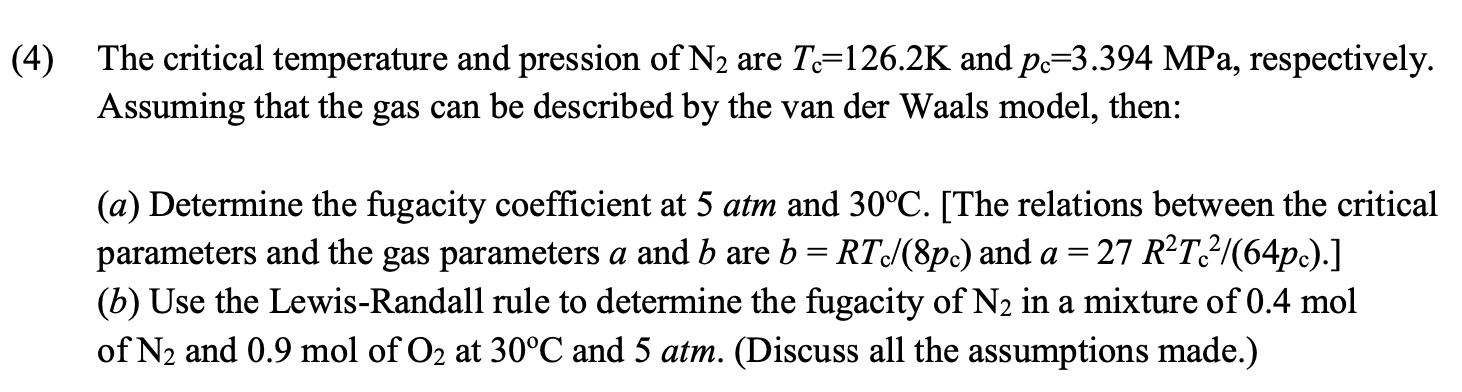
( 4 ) The critical temperature and pression of N 2 are T c = 1 2 6 . 2 K and p c =
The critical temperature and pression of are and MPa, respectively.
Assuming that the gas can be described by the van der Waals model, then:
a Determine the fugacity coefficient at atm and The relations between the critical
parameters and the gas parameters a and are and
b Use the LewisRandall rule to determine the fugacity of in a mixture of mol
of and mol of at and atm. Discuss all the assumptions made.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


