Question
4. The following data were obtained for the irreversible, gas-phase reaction: A+ BR+S using an isothermal, ideal plug- flow reactor. The feed contained a
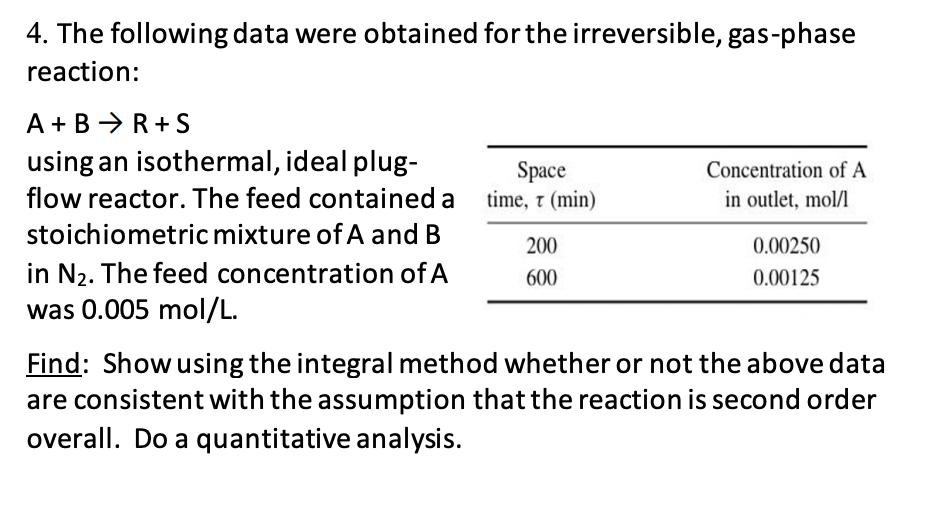
4. The following data were obtained for the irreversible, gas-phase reaction: A+ BR+S using an isothermal, ideal plug- flow reactor. The feed contained a stoichiometric mixture of A and B in N. The feed concentration of A was 0.005 mol/L. Space time, 7 (min) 200 600 Concentration of A in outlet, mol/l 0.00250 0.00125 Find: Show using the integral method whether or not the above data are consistent with the assumption that the reaction is second order overall. Do a quantitative analysis.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus
Authors: Ron Larson, Bruce H. Edwards
10th Edition
1285057090, 978-1285057095
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



