Question
4.1. Which two salts display discontinuities in their solubility curves? Explain what contributes to the occurrence of these discontinuities? 4.2. Which form of salt will
4.1. Which two salts display discontinuities in their solubility curves? Explain what contributes to the occurrence of these discontinuities?
4.2. Which form of salt will be stable at a solubility of 160 kg of FeSO4 /100 kg water and 72°C?
4.3. What is the mass fraction of FeSO4 present in a saturated solution of FeSO4 at 27°C?
4.4. About 100 kg of water is added to a beaker containing 82 kg of Na2HPO4 and is continuously stirred to prepare a solution at 49°C. (use figure 1) 4.4.1. What type of solution (saturated, unsaturated, supersaturated) will be present at this temperature?
4.4.2. If the temperature of the solution is suddenly reduced to 40.5°C. How many grams of solute will precipitate out of the solution?
4.4.3. What is the chemical formula of the crystals which will form in question 4.4.2?
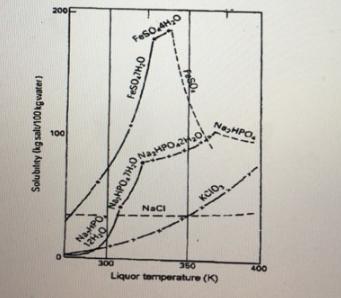
4.1. Which two salts display discontinuities in their solubility curves? Explain what contributes to the occurrence of these discontinuities? (3)
4.2. Which form of salt will be stable at a solubility of 160 kg of FeSO4 /100 kg water and 72°C? (1)
4.3. What is the mass fraction of FeSO4 present in a saturated solution of FeSO4 at 27°C? (4)
4.4. About 100 kg of water is added to a beaker containing 82 kg of Na2HPO4 and is continuously stirred to prepare a solution at 49°C. (use figure 1) 4.4.1. What type of solution (saturated, unsaturated, supersaturated) will be present at this temperature? (1)
4.4.2. If the temperature of the solution is suddenly reduced to 40.5°C. How many grams of solute will precipitate out of the solution? (3)
4.4.3. What is the chemical formula of the crystals which will form in question 4.4.2? (14.1. Which two salts display discontinuities in their solubility curves? Explain what contributes to the occurrence of these discontinuities? (3)
4.2. Which form of salt will be stable at a solubility of 160 kg of FeSO4 /100 kg water and 72°C? (1)
4.3. What is the mass fraction of FeSO4 present in a saturated solution of FeSO4 at 27°C? (4)
4.4. About 100 kg of water is added to a beaker containing 82 kg of Na2HPO4 and is continuously stirred to prepare a solution at 49°C. (use figure 1) 4.4.1. What type of solution (saturated, unsaturated, supersaturated) will be present at this temperature? (1)
4.4.2. If the temperature of the solution is suddenly reduced to 40.5°C. How many grams of solute will precipitate out of the solution? (3)
4.4.3. What is the chemical formula of the crystals which will form in question 4.4.2? (1)
200 FeSO AHO 100 NaHPO. NaCI KCIO, 12H,0 300 350 Liquor temperature (K) Solubitey (kg salu100 kgvwater)
Step by Step Solution
3.46 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


