
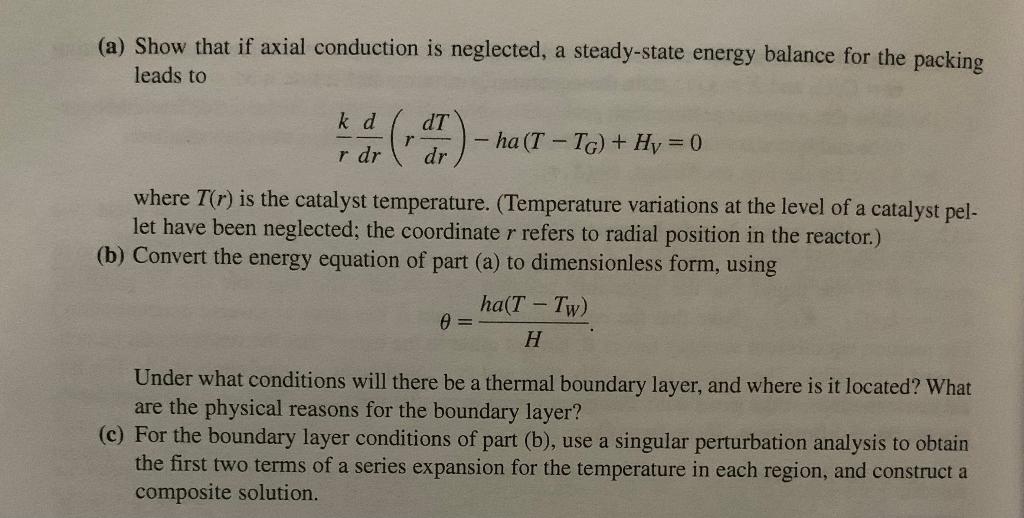
4-14. Heat Transfer in a Packed Bed Reactor Suppose that a long, cylindrical reactor of radius R is packed with catalyst pellets. Gas flow through the packed bed supplies a component that reacts exothermically at the surface of the pellets, releasing heat at a constant volumetric rate HV. The pellet surface area per unit volume is a and heat transfer from the pellets to the gas is characterized by a heat transfer coefficient h. For simplicity, the bulk gas temperature TG and reactor wall temperature TW are each assumed to be constant. The packing has an effective thermal conductivity k. (a) Show that if axial conduction is neglected, a steady-state energy balance for the packing leads to rkdrd(rdrdT)ha(TTG)+HV=0 where T(r) is the catalyst temperature. (Temperature variations at the level of a catalyst pellet have been neglected; the coordinate r refers to radial position in the reactor.) (b) Convert the energy equation of part (a) to dimensionless form, using =Hha(TTW) Under what conditions will there be a thermal boundary layer, and where is it located? What are the physical reasons for the boundary layer? (c) For the boundary layer conditions of part (b), use a singular perturbation analysis to obtain the first two terms of a series expansion for the temperature in each region, and construct a composite solution. 4-14. Heat Transfer in a Packed Bed Reactor Suppose that a long, cylindrical reactor of radius R is packed with catalyst pellets. Gas flow through the packed bed supplies a component that reacts exothermically at the surface of the pellets, releasing heat at a constant volumetric rate HV. The pellet surface area per unit volume is a and heat transfer from the pellets to the gas is characterized by a heat transfer coefficient h. For simplicity, the bulk gas temperature TG and reactor wall temperature TW are each assumed to be constant. The packing has an effective thermal conductivity k. (a) Show that if axial conduction is neglected, a steady-state energy balance for the packing leads to rkdrd(rdrdT)ha(TTG)+HV=0 where T(r) is the catalyst temperature. (Temperature variations at the level of a catalyst pellet have been neglected; the coordinate r refers to radial position in the reactor.) (b) Convert the energy equation of part (a) to dimensionless form, using =Hha(TTW) Under what conditions will there be a thermal boundary layer, and where is it located? What are the physical reasons for the boundary layer? (c) For the boundary layer conditions of part (b), use a singular perturbation analysis to obtain the first two terms of a series expansion for the temperature in each region, and construct a composite solution








