Answered step by step
Verified Expert Solution
Question
1 Approved Answer
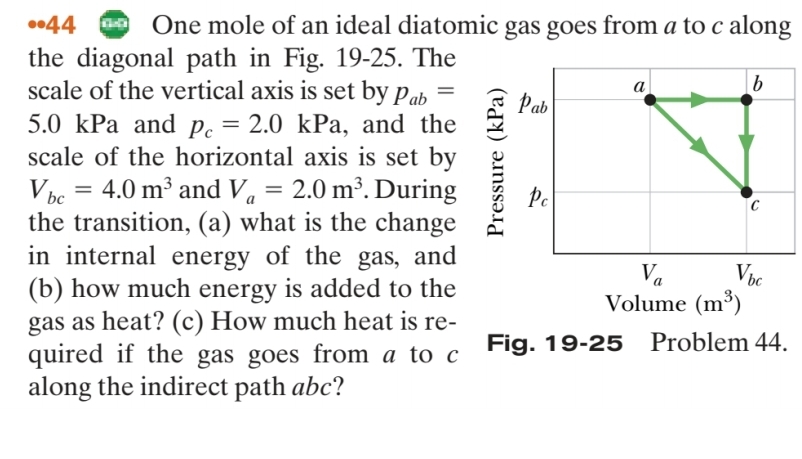
-44 One mole of an ideal diatomic gas goes from a to c along the diagonal path in Fig. 19-25. The scale of the vertical
-44 One mole of an ideal diatomic gas goes from
a to
c along\ the diagonal path in Fig. 19-25. The\ scale of the vertical axis is set by
p_(ab)=\
5.0kPa and
p_(c)=2.0kPa, and the\ scale of the horizontal axis is set by\
V_(bc)=4.0m^(3) and
V_(a)=2.0m^(3). During\ the transition, (a) what is the change\ in internal energy of the gas, and\ (b) how much energy is added to the\ gas as heat? (c) How much heat is re-\ quired if the gas goes from
a to
c\ Fiy. iv-c0 rivuicilit4.\ along the indirect path
abc ?
 - 44 One mole of an ideal diatomic gas goes from a to c along the diagonal path in Fig. 19-25. The scale of the vertical axis is set by pab= 5.0kPa and pc=2.0kPa, and the scale of the horizontal axis is set by Vbc=4.0m3 and Va=2.0m3. During the transition, (a) what is the change in internal energy of the gas, and (b) how much energy is added to the gas as heat? (c) How much heat is required if the gas goes from a to c Fig. 19-25 Problem 44. along the indirect path abc
- 44 One mole of an ideal diatomic gas goes from a to c along the diagonal path in Fig. 19-25. The scale of the vertical axis is set by pab= 5.0kPa and pc=2.0kPa, and the scale of the horizontal axis is set by Vbc=4.0m3 and Va=2.0m3. During the transition, (a) what is the change in internal energy of the gas, and (b) how much energy is added to the gas as heat? (c) How much heat is required if the gas goes from a to c Fig. 19-25 Problem 44. along the indirect path abc
-44 One mole of an ideal diatomic gas goes from
ato
calong\ the diagonal path in Fig. 19-25. The\ scale of the vertical axis is set by
p_(ab)=\
5.0kPaand
p_(c)=2.0kPa, and the\ scale of the horizontal axis is set by\
V_(bc)=4.0m^(3)and
V_(a)=2.0m^(3). During\ the transition, (a) what is the change\ in internal energy of the gas, and\ (b) how much energy is added to the\ gas as heat? (c) How much heat is re-\ quired if the gas goes from
ato
c\ Fiy. iv-c0 rivuicilit4.\ along the indirect path
abc?

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


