Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Assume the following process is steady-state. 4,500 kg/h of a solution that is 1/3 K2Cro, by mass is joined by a recycle stream containing
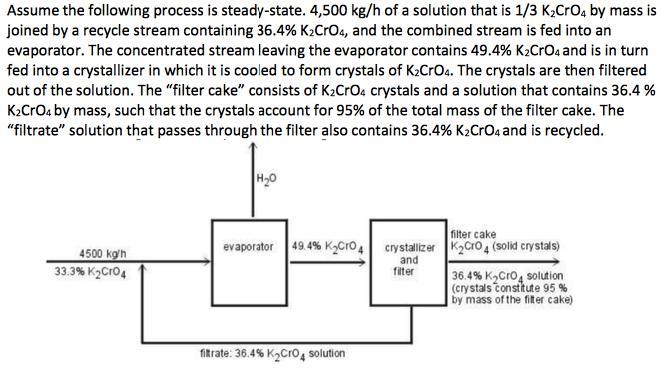

Assume the following process is steady-state. 4,500 kg/h of a solution that is 1/3 K2Cro, by mass is joined by a recycle stream containing 36.4% K2CrO4, and the combined stream is fed into an evaporator. The concentrated stream leaving the evaporator contains 49.4% K2CrOa and is in turn fed into a crystallizer in which it is cooled to form crystals of K2CrOa. The crystals are then filtered out of the solution. The "filter cake" consists of K2CrOa crystals and a solution that contains 36.4 % K2CrOa by mass, such that the crystals account for 95% of the total mass of the filter cake. The "filtrate" solution that passes through the filter also contains 36.4% K2Croa and is recycled. H20 filter cake evaporator 49.4% KgCro, crystalizer K2cro4 (solid crystals) 4500 ko'h 33.3% K2Cro4 and fiter 36.4% K,Cro, solution (crystals constkute 95 % by mass of the fiter cake) fitrate: 36.4% K2Cro4 solution a. What are: (i) mass rate of evaporation, (ii) the mass rate of production of K2CrO4 crystals, (iii) the mass feed rates that the evaporator and the crystallizer must handle, and (iv) the recycle ratio? b. What would be the mass production rate of the crystals if the filtrate was discarded instead of recycled?
Step by Step Solution
★★★★★
3.46 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


