Question
4G l 10:05 Vo) LT 77 epraghna.com/student, 3 Lattice energy is the energy released, when one mole of ionic solid is formed from its
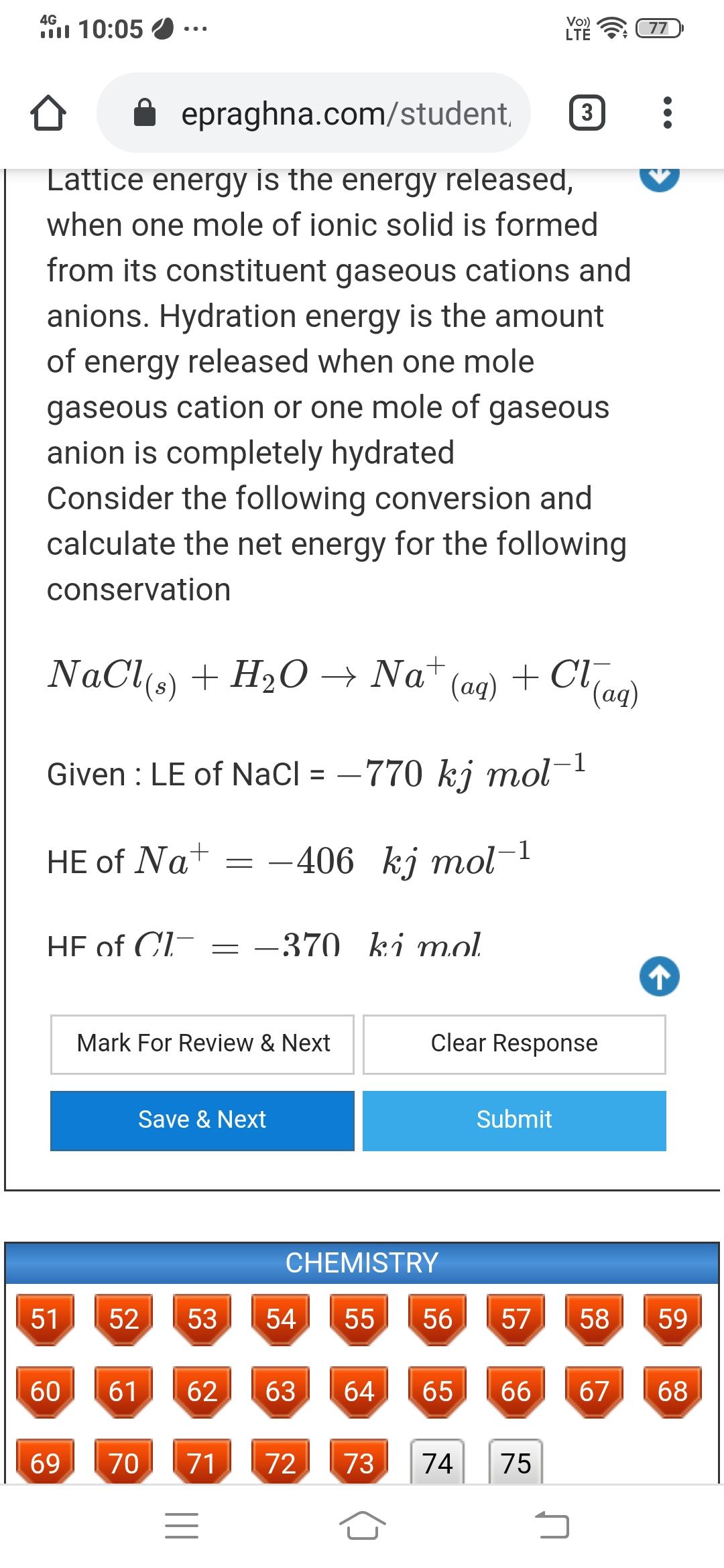
4G l 10:05 Vo) LT 77 epraghna.com/student, 3 Lattice energy is the energy released, when one mole of ionic solid is formed from its constituent gaseous cations and anions. Hydration energy is the amount of energy released when one mole gaseous cation or one mole of gaseous anion is completely hydrated Consider the following conversion and calculate the net energy for the following conservation NaCls) + H2O Na* (ag) + Clan) (ag) Given : LE of NaCl = 770 kj mol-1 HE of Na+ -406 kj mol-1 HE of Cl- = -370 ki mol. Mark For Review & Next Clear Response Save & Next Submit CHEMISTRY 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of biochemistry Life at the Molecular Level
Authors: Donald Voet, Judith G. Voet, Charlotte W. Pratt
4th edition
470547847, 978-0470547847
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



