Answered step by step
Verified Expert Solution
Question
1 Approved Answer
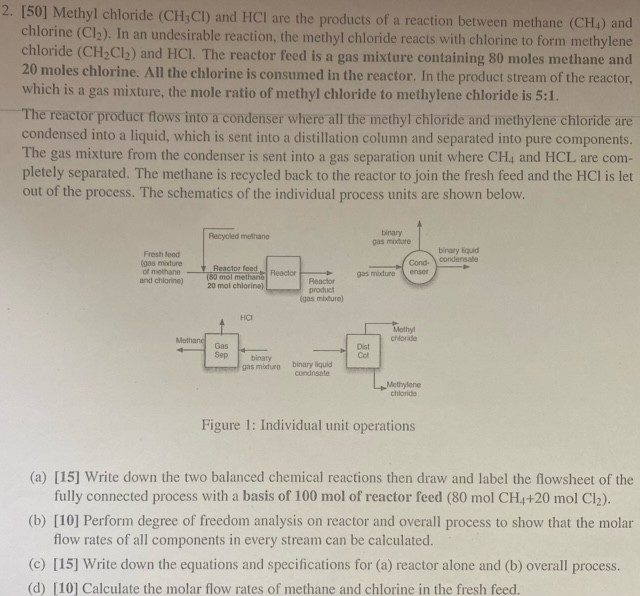
[ 5 0 ] Methyl chloride ( C H 3 C l ) and H C l are the products of a reaction between methane
Methyl chloride and are the products of a reaction between methane and chlorine In an undesirable reaetion, the methyl chloride reacts with chlorine to form methylene chloride and The reactor feed is a gas mixture containing moles methane and moles chlorine. All the chlorine is consumed in the reactor. In the product stream of the reactor, which is a gas mixture, the mole ratio of methyl chloride to methylene chloride is :
The reactor product flows into a condenser where all the methyl chloride and methylene chloride are condensed into a liquid, which is sent into a distillation column and separated into pure components. The gas mixture from the condenser is sent into a gas separation unit where and are completely separated. The methane is recycled back to the reactor to join the fresh feed and the is let out of the process. The schematics of the individual process units are shown below.
Figure : Individual unit operations
a Write down the two balanced chemical reactions then draw and label the flowsheet of the fully connected process with a basis of mol of reactor feed
b Perform degree of freedom analysis on reactor and overall process to show that the molar flow rates of all components in every stream can be calculated.
c Write down the equations and specifications for a reactor alone and b overall process.
d Calculate the molar flow rates of methane and chlorine in the fresh feed.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


