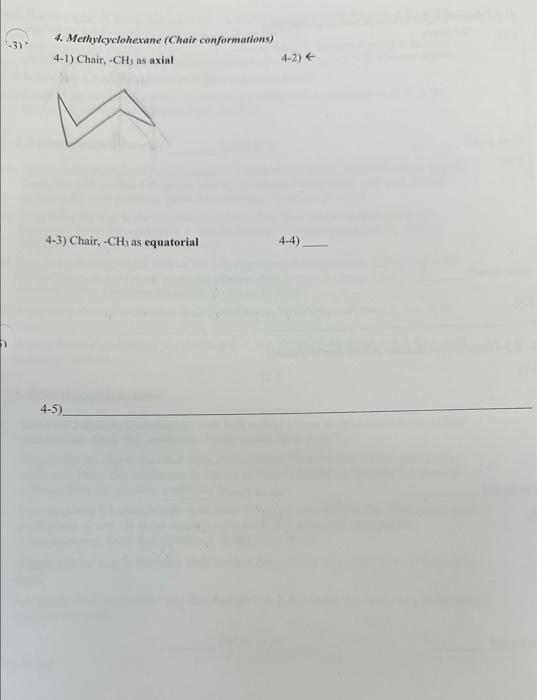
5. 1,2-Dimethylavlohexane (Chair corformatlons) 5-1) 5-2) cis or thans? cis or tronts? 5-3) \$.4) chs of trans? cis or thuns? 5-5) 4. Methy/cyclohexane (Chair conformations) 4-1) Chair, CH3 as axial 42) 4-3) Chair, - CH3 as equatorial 4-4) 4-2) Find two axial - H groups that cause the 1,3-diaxial interactions with the axial CH3 group. Mark these 1,3-diaxial interactions on your drawing in 4-1. 4-3) Now wiggle/fip the ring to another chair conformation with the methyl group at an equtyil position. Draw this conformer with all axial and equatorial H groups with accurate angles. 4-4) Is there any 1,3-diaxial interaction in this conformer? Yes/No 4-5) Based on the examination above, explain which conformation, equatorial or axial, is the most stable (lowest in potential energy), and why. 5. 1,2-Dimethylcyclohexane 5-1) To your axial methylcyclohexane model in a chair conformation, replace the H atom at the 2-position with another CH3 group. This is 1,2-dimethylcyclohexane with both methyl groups at the axial positions. Draw this conformer. Is this c is or trans? 5-2) Wiggle/flip the ring to the other chair conformation. Now the two methyl groups are equatorial. Draw this conformer. Is this cis or trans? (Should cis/trans be the same or different from the previous conformer?) 5-3) Now from ring Cl, detach both CH3 and H groups, and reattach the CH3 group at the axial position, and H at the equatorial position. You have now changed the stereochemistry. Draw this conformer. Is this cis or trans? 5-4) Wiggle/flip the ring to the other chair conformation. Draw this conformer. Is this cis or trans? 5-5) Among the four conformers you sketched (5-1, 5-2,5-3 and 5-4), which one is the lowest. in energy? Explain. 6. 1,3-DiMethylcyclohexane 6-1) Build a 1,3-dimethylcyclohexane, with both methyl groups at axial positions in a chaircyclohexane. Draw this conformer. Is this isomer cis or trans? 6-2) Wiggle/flip the ring to the other chair conformation. Now the two methyl groups are equatorial. Draw this conformer. Is this cis or trans? (Should cis/trans be the same or different from the previous conformer?) 6-3) Now from ring Cl, detach both CH3 and H groups, and reattach the CH3 group at the axial position, and H at the equatorial position. You have now changed the stereochemistry. Draw this conformer. Is this cis or trans? 6-4) Wiggle/flip the ring to the other chair conformation. Draw this conformer. Is this cis or trans? 6-5) Among the four conformers you sketched (61,62,63 and 6-4), which one is the lowest in energy? Explain. 5. 1,2-Dimethylavlohexane (Chair corformatlons) 5-1) 5-2) cis or thans? cis or tronts? 5-3) \$.4) chs of trans? cis or thuns? 5-5) 4. Methy/cyclohexane (Chair conformations) 4-1) Chair, CH3 as axial 42) 4-3) Chair, - CH3 as equatorial 4-4) 4-2) Find two axial - H groups that cause the 1,3-diaxial interactions with the axial CH3 group. Mark these 1,3-diaxial interactions on your drawing in 4-1. 4-3) Now wiggle/fip the ring to another chair conformation with the methyl group at an equtyil position. Draw this conformer with all axial and equatorial H groups with accurate angles. 4-4) Is there any 1,3-diaxial interaction in this conformer? Yes/No 4-5) Based on the examination above, explain which conformation, equatorial or axial, is the most stable (lowest in potential energy), and why. 5. 1,2-Dimethylcyclohexane 5-1) To your axial methylcyclohexane model in a chair conformation, replace the H atom at the 2-position with another CH3 group. This is 1,2-dimethylcyclohexane with both methyl groups at the axial positions. Draw this conformer. Is this c is or trans? 5-2) Wiggle/flip the ring to the other chair conformation. Now the two methyl groups are equatorial. Draw this conformer. Is this cis or trans? (Should cis/trans be the same or different from the previous conformer?) 5-3) Now from ring Cl, detach both CH3 and H groups, and reattach the CH3 group at the axial position, and H at the equatorial position. You have now changed the stereochemistry. Draw this conformer. Is this cis or trans? 5-4) Wiggle/flip the ring to the other chair conformation. Draw this conformer. Is this cis or trans? 5-5) Among the four conformers you sketched (5-1, 5-2,5-3 and 5-4), which one is the lowest. in energy? Explain. 6. 1,3-DiMethylcyclohexane 6-1) Build a 1,3-dimethylcyclohexane, with both methyl groups at axial positions in a chaircyclohexane. Draw this conformer. Is this isomer cis or trans? 6-2) Wiggle/flip the ring to the other chair conformation. Now the two methyl groups are equatorial. Draw this conformer. Is this cis or trans? (Should cis/trans be the same or different from the previous conformer?) 6-3) Now from ring Cl, detach both CH3 and H groups, and reattach the CH3 group at the axial position, and H at the equatorial position. You have now changed the stereochemistry. Draw this conformer. Is this cis or trans? 6-4) Wiggle/flip the ring to the other chair conformation. Draw this conformer. Is this cis or trans? 6-5) Among the four conformers you sketched (61,62,63 and 6-4), which one is the lowest in energy? Explain