Answered step by step
Verified Expert Solution
Question
1 Approved Answer
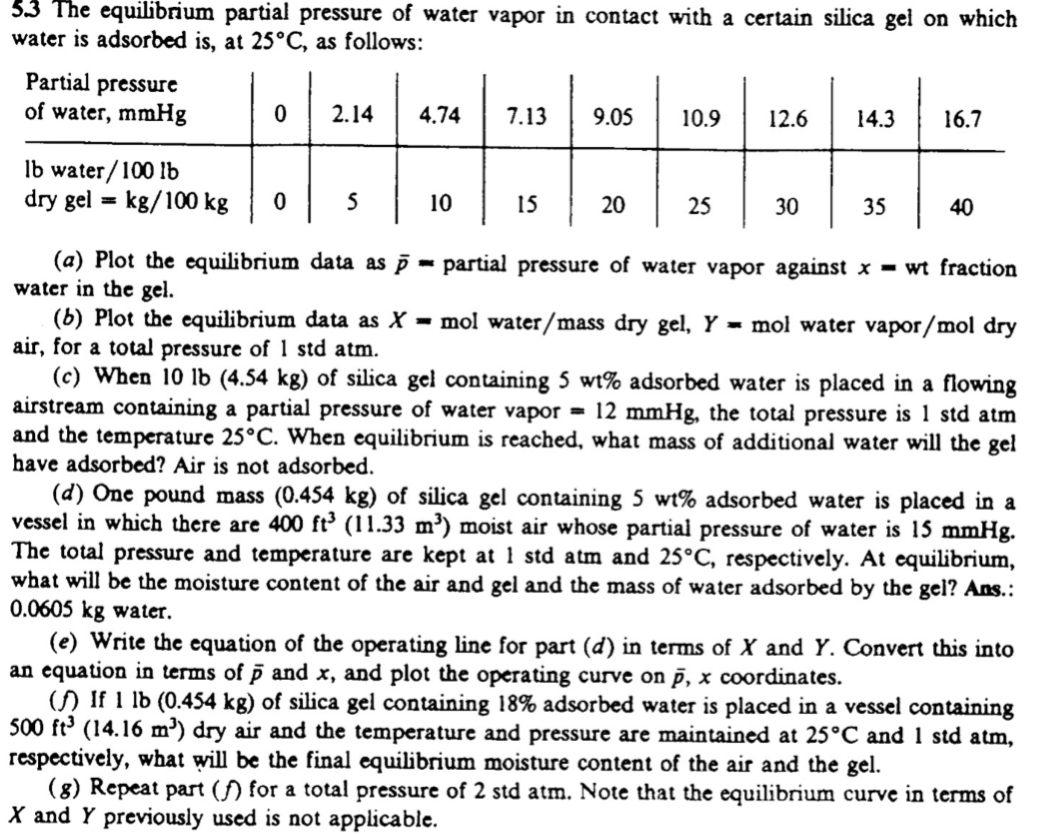
5 . 3 The equilibrium partial pressure of water vapor in contact with a certain silica gel on which water is adsorbed is , at
The equilibrium partial pressure of water vapor in contact with a certain silica gel on which
water is adsorbed is at as follows:
a Plot the equilibrium data as partial pressure of water vapor against wt fraction
water in the gel.
b Plot the equilibrium data as mol water dry gel, mol water vapor
air, for a total pressure of atm.
c When of silica gel containing adsorbed water is placed in a flowing
airstream containing a partial pressure of water vapor the total pressure is atm
and the temperature When equilibrium is reached, what mass of additional water will the gel
have adsorbed? Air is not adsorbed.
d One pound mass of silica gel containing adsorbed water is placed in a
vessel in which there are moist air whose partial pressure of water is
The total pressure and temperature are kept at atm and respectively. At equilibrium,
what will be the moisture content of the air and gel and the mass of water adsorbed by the gel? Ans.:
water.
e Write the equation of the operating line for part d in terms of and Convert this into
an equation in terms of and and plot the operating curve on coordinates.
If of silica gel containing adsorbed water is placed in a vessel containing
dry air and the temperature and pressure are maintained at and stdatm,
respectively, what will be the final equilibrium moisture content of the air and the gel.
Repeat part for a total pressure of atm. Note that the equilibrium curve in terms of
and previously used is not applicable.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


